4-氨基苯酚硫酸盐 | 15658-52-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1.606±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:98
-
氢给体数:2
-
氢受体数:5
安全信息
-
海关编码:2922199090
SDS
反应信息
-
作为反应物:描述:4-氨基苯酚硫酸盐 在 mineral acid 作用下, 生成 1-((4-hydroxyphenyl)diazenyl)naphthalen-2-ol参考文献:名称:高能机械研磨制备的氧化铈-氧化锆粉末催化剂的结构表征:中子衍射研究摘要:对通过高能机械研磨制备的 CeO 2 –ZrO 2 粉末催化剂样品进行中子衍射测量。在所检查的整个组成范围内都证明了固溶体的形成。通过 Rietveld 方法进行的定量相评估表明,在低 CeO 2 含量下形成四方结构,而在高 CeO 2 负载下立方固溶体是稳定形式。此外,在中间组成(50 mol% CeO 2 )下观察到具有统一轴比和氧亚晶格内部变形的假立方或四方t”电池。高达 1000 °C 的热退火显示了晶胞参数的膨胀;对于成分 Ce x Zr 1− x O 2 ( xDOI:10.1557/jmr.2000.0220
-
作为产物:描述:参考文献:名称:Engel, Journal of the American Chemical Society, 1929, vol. 51, p. 2992摘要:DOI:
文献信息
-
Synthesis of Copper and Lithium Copper Ferrites as High Magnetization Materials作者:K. E. Kuehn、D. Sriram、S. S. Bayya、J. J. Simmins、R. L. SnyderDOI:10.1557/jmr.2000.0235日期:2000.7
The ferrite with composition Cu0.5Fe2.5O4 was heat treated in air and in reducing atmospheres to different temperatures within the solid solution region confirmed by dynamic high-temperautre x-ray characterization. The samples were quenched in oil and air, and lattice parameter, Curie temperature, and saturation magnetization measurements were completed. The magnetization measurements for these samples showed a maximum 4π
M s of 0.7729 and 0.5426 T at 10 and 300 K, respectively. The cationic distribution based on the low-temperature 4πM s measurements is (Cu+0.24Fe3+0.76)A[Cu+0.26Fe3+1.74]BO4 → 4.9 µ B. X-ray-pure Cu0.5Fe2.5O4 samples were also synthesized by slow cooling from the formation temperature to 900 °C in a reducing atmosphere. A temperature–P O2 diagram for the stability of Cu0.5Fe2.5O4 under the conditions of the experiment was determined. Low-temperature 4πM s measurements did not indicate an increase in the Cu+ A site occupancy for the samples cooled to 900 °C in a reducing environment above those samples that were quenched from high temperature. Curie temperatures for all Cu0.5Fe2.5O4 samples ranged from 348 to 369 °C. Lithium additions (0.1 mol/unit formula) to copper ferrite Li0.1Cu0.4Fe2.5O4 decreased the room-temperature 4πM s values to 0.5234 T with a corresponding decrease in the 10 K measurements to 0.7047 T. From the low-temperature magnetization measurements, the distribution was (Cu+0.15Fe3+0.85)A[Cu+0.25Li+0.1Fe3+1.65]BO4 → 4.48 µ B.在空气和还原气氛中将成分为 Cu0.5Fe2.5O4 的铁氧体热处理到固溶体区域内的不同温度,并通过动态高温 X 射线表征加以确认。样品在油和空气中淬火后,完成了晶格参数、居里温度和饱和磁化测量。这些样品的磁化测量结果显示,在 10 K 和 300 K 时,最大 4πMs 分别为 0.7729 和 0.5426 T。根据低温 4πMs 测量结果,阳离子分布为 (Cu+0.24Fe3+0.76)A[Cu+0.26Fe3+1.74]BO4 → 4.9 µ B。X-射线纯 Cu0.5Fe2.5O4 样品也是在还原气氛中从形成温度缓慢冷却至 900 ℃ 合成的。确定了 Cu0.5Fe2.5O4 在实验条件下的稳定性温度-PO2 图。低温 4πMs 测量结果表明,在还原环境中冷却到 900 ℃ 的样品的 Cu+ A 位点占有率并没有比高温淬火的样品增加。所有 Cu0.5Fe2.5O4 样品的居里温度都在 348 ℃ 至 369 ℃ 之间。在铜铁氧体 Li0.1Cu0.4Fe2.5O4 中添加锂(0.1 摩尔/单位配方)后,室温 4πMs 值降至 0.5234 T,10 K 测量值相应降至 0.7047 T。 -
BIS-ARYL COMPOUNDS FOR USE AS MEDICAMENTS申请人:Nilsson Peter公开号:US20110071197A1公开(公告)日:2011-03-24There is provided compounds of formula I, wherein ring A, D 1 , D 2a , D 2b , D 3 , L 1 , Y 1 , L 3 and Y 3 have meanings given in the description, and pharmaceutically-acceptable salts thereof, which compounds are useful in the treatment of diseases in which inhibition of leukotriene C 4 synthase is desired and/or required, and particularly in the treatment of a respiratory disorder and/or inflammation.提供了公式I的化合物,其中环A,D1,D2a,D2b,D3,L1,Y1,L3和Y3具有描述中给出的含义,并且其药学上可接受的盐,这些化合物在治疗需要和/或需要抑制白三烯C4合酶的疾病方面是有用的,特别是在治疗呼吸道疾病和/或炎症方面。
-
Azofarbstoffe, deren Herstellung und Verwendung申请人:CIBA-GEIGY AG公开号:EP0036838A2公开(公告)日:1981-09-30Azofarbstoffe der Formel worin D der Rest einer aromatischen Diazokomponente, der weitersubstituiert sein kann und selbst eine Azobrücke enthalten kann, und der eine Halogen-s-triazinylaminogruppe enthält, und R Wasserstoff oder Alkyl mit 1 bis 4 Kohlenstoffatomen ist, und R1 und R2 unabhängig voneinander nichtreaktive Substituenten sind. Die Azofarbstoffe der Formel (1) eignen sich besonders für das Färben von Baumwollgewebe nach dem Ausziehverfahren. Die Färbungen zeichnen sich durch gute Echtheitseigenschaften aus.
-
Bis-1:2-chromkomplexe von Disazofarbstoffen, deren Herstellung und Verwendung申请人:CIBA-GEIGY AG公开号:EP0100297A1公开(公告)日:1984-02-08Die Farbstoffe der Formel I worin A, B, C, D, E, F, Z, Z', X, X', Y, n, n1, n2 und n3, p und Ka⊕ die im Anspruch 1 angegebene Bedeutung aufweisen, eignen sich insbesondere zum Färben von Wolle oder Polyamid und vor allem für Leder.式 I 的着色剂 其中 A、B、C、D、E、F、Z、Z'、X、X'、Y、n、n1、n2 和 n3、p 和 Ka⊕ 具有权利要求 1 中的含义,特别适用于羊毛或聚酰胺染色,尤其适用于皮革染色。
-
Polyaniline-containing solution and method for preparing the same申请人:Conpoly Technology Co., Ltd公开号:EP1132415A1公开(公告)日:2001-09-12A process for producing a conductive polyaniline is disclosed. The process includes mixing an aniline monomer, a water-immiscible organic solvent, at least one protonic acid doping agent and water together to form an emulsion via agitation, stopping agitation to allow the emulsion to separate into an aqueous phase and a non-aqueous phase, separating the non-aqueous phase from the aqueous phase, and adding an oxidation agent into the non-aqueous phase to carry out polymerization. A non-aqueous in-situ formed transparent polyaniline solution prepared by the process is also disclosed.
表征谱图
-
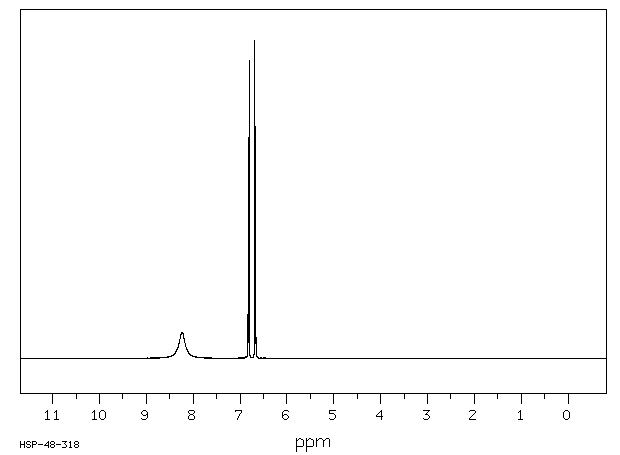
氢谱1HNMR
-
质谱MS
-
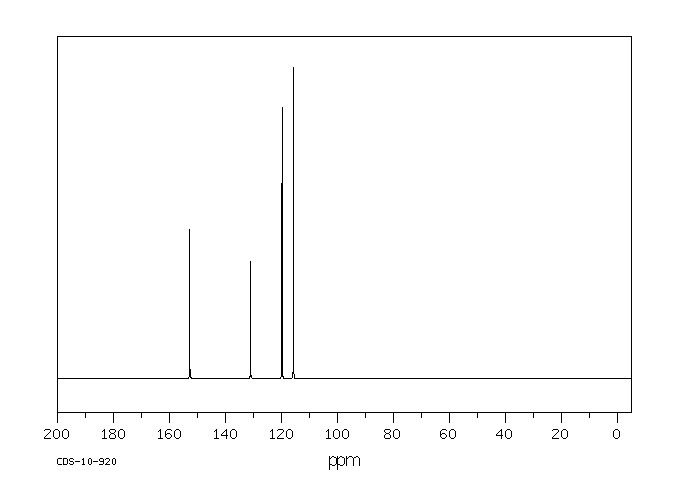
碳谱13CNMR
-
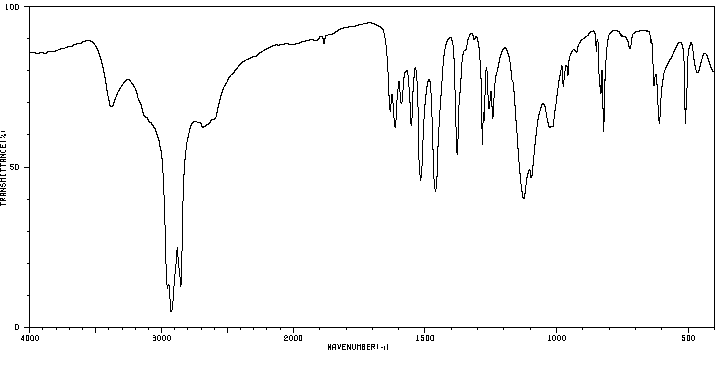
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息