3,4,4,5-四甲基-4H-吡唑 | 19078-32-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:59 °C
-
沸点:126 °C(Press: 26 Torr)
-
密度:0.9274 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:24.7
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933199090
SDS
反应信息
-
作为反应物:描述:3,4,4,5-四甲基-4H-吡唑 在 10percent Pd-C 硫酸 、 氢气 、 potassium carbonate 、 三乙胺 、 三氟乙酸 、 肼 作用下, 以 甲醇 、 二氯甲烷 、 水 、 乙腈 为溶剂, -78.0~65.0 ℃ 、1350.02 MPa 条件下, 反应 400.5h, 生成 (2R*,3S*,5S*,6S*)-2,16,17,17-tetramethyl-1,12,13,15-tetraazaheptacyclo[10.5.1.01,15.03,11.04,16.05,13.06,10]octadecane参考文献:名称:Exner, Kai; Fischer, Gerhard; Bahr, Nikolaus, European Journal of Organic Chemistry, 2000, # 5, p. 763 - 785摘要:DOI:
-
作为产物:描述:参考文献:名称:5-羟基-4,5-二氢吡唑摘要:在与肼及其单取代衍生物的反应中使用具有强吸电子取代基的β-二酮可生成吡唑合成的稳定中间体-5-羟基-4.5-二氢吡唑或其开链异构体。DOI:10.1016/0040-4020(95)00672-u
文献信息
-
Diastereo- and Regioselective Synthesis of Diquinanes and Related Systems from Tricyclo[3.3.0.02,4]octanes by Chemical Electron Transfer (CET)作者:Waldemar Adam、Thomas Heidenfelder、Coskun SahinDOI:10.1055/s-1995-4072日期:1995.9A new synthetic methodology for diquinanes by one-electron oxidation of tricyclo[3.3.0.02,4]octanes and subsequent stereocontrolled rearrangement is provided. The latter compounds are conveniently accessible through acid-catalyzed isopyrazole cycloaddition, followed by hydrogenation and photoextrusion of molecular nitrogen. The oxidative rearrangement of the tricyclooctanes proceeds catalytically and cleanly to afford regio- and diastereoselectively the corresponding diquinanes.
-
Treatment of Tetramethylpyrazole and 3,5-Diphenylpyrazole with Dialkylaluminum Hydrides - Hydroalumination versus Deprotonation作者:Werner Uhl、Andreas VogelpohlDOI:10.1515/znb-2010-0604日期:2010.6.1
Treatment of tetramethylpyrazole, N2C3Me4, with equimolar quantities of di(tert-butyl)aluminum hydride leads to the addition of an Al-H bond to one of the C=N double bonds. The dimeric product (1) contains a central six-membered Al2N4 ring in which two tBu2Al+ units are bridging two N2C3 heterocycles. In the zwitterionic, non-centrosymmetric compound one aluminum atom is coordinated by two imino nitrogen atoms, while the second one is bonded to two amide nitrogen atoms. No double hydroalumination occurs upon treatment of tetramethylpyrazole with two equivalents of the hydride. Instead, an adduct (2) of the monomeric hydroalumination product with di(tert-butyl)aluminum hydride was isolated in which the two aluminum atoms are connected by a 3c-2e Al-H-Al bond. A unique trinuclear compound (3) is obtained upon reaction of tetramethylpyrazole with an excess of the sterically less shielded diethylaluminum hydride. It contains two different N2C3 heterocycles: One still contains a C=N double bond similar to that in compounds 1 and 2, while the second one is completely reduced by double hydroalumination to give a saturated heterocycle. The two rings are bridged by three AlEt2 groups. Deprotonation results upon treatment of 3,5-diphenylpyrazole, N2C3H2(C6H5)2, with di(tert-butyl)aluminum hydride.
将四甲基吡唑基N2C3Me4与等量的叔丁基铝氢反应,会将一个C=N双键中的一个Al-H键加成。二聚体产物(1)包含一个中心六元环Al2N4环,其中两个tBu2Al+单元桥接两个N2C3杂环。在这种带电离的非中心对称化合物中,一个铝原子被两个亚胺氮原子配位,而第二个铝原子则与两个酰胺氮原子键合。当将两当量的氢化物与四甲基吡唑处理时,不会发生双重氢铝化反应。相反,分离出了单体氢铝化产物与叔丁基铝氢的加合物(2),其中两个铝原子通过3c-2e Al-H-Al键连接。当将四甲基吡唑与过量的空间位阻较小的二乙基铝氢反应时,会得到一种独特的三核化合物(3)。它包含两个不同的N2C3杂环:一个仍然含有类似于化合物1和2中的C=N双键,而第二个则通过双重氢铝化完全还原为饱和杂环。这两个环通过三个AlEt2基团桥接。在将3,5-二苯基吡唑基N2C3H2(C6H5)2与叔丁基铝氢反应时,会发生去质子化反应。 -
Isopyrazole‐Masked Tetraketone: Tautomerism and Functionalization for Fluorescent Metal Ligands作者:Hayato Shirakura、Yumehiro Manabe、Chika Kasai、Yuya Inaba、Makoto Tsurui、Yuichi Kitagawa、Yasuchika Hasegawa、Tomoki Yoneda、Yuki Ide、Yasuhide InokumaDOI:10.1002/ejoc.202100784日期:2021.8.13masked-tetraketone has six possible tautomers, but exists predominantly as a 12π-electron conjugated keto–enamine–imine–enol form, which was also observed in the solid state by single crystal X-ray analysis. Stepwise functionalization to introduce metal-coordination and fluorescence-enhancement sites into the masked tetraketone furnished a fluorescent chromophore that indicates metal coordination on a microgram
-
Stephanidou-Stephanatou, J., Journal of Heterocyclic Chemistry, 1983, vol. 20, p. 845 - 853作者:Stephanidou-Stephanatou, J.DOI:——日期:——
-
Baumstark, Alfons L.; Choudhary, Anil; Vasquez, Pedro C., Journal of Heterocyclic Chemistry, 1990, vol. 27, # 2, p. 291 - 294作者:Baumstark, Alfons L.、Choudhary, Anil、Vasquez, Pedro C.、Dotrong, MyDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
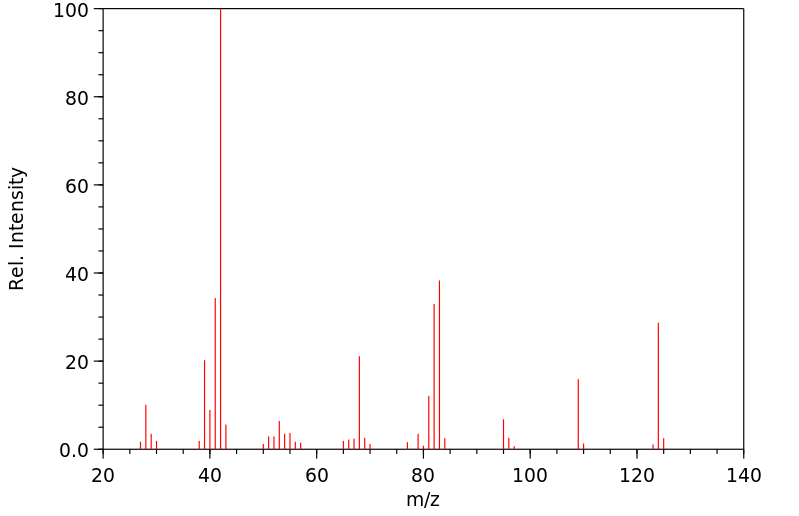
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息