3-羟基-2-甲基-1-丙基吡啶-4-酮 | 30652-13-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:266.0±40.0 °C(Predicted)
-
密度:1.132±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.44
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-benzyloxy-2-methyl-1-n-propyl-4(1H)-pyridinone 90037-24-4 C16H19NO2 257.332
反应信息
-
作为反应物:描述:3-羟基-2-甲基-1-丙基吡啶-4-酮 以 二氯甲烷 为溶剂, 反应 12.0h, 生成 1,4-dihydro-2-methyl-1-propyl-4-propylimino-3-pyridinol参考文献:名称:Faerber, M.; Osiander, H.; Severin, T., Journal of Heterocyclic Chemistry, 1994, vol. 31, # 4, p. 947 - 956摘要:DOI:
-
作为产物:描述:参考文献:名称:Efficient Syntheses of N-Alkyl-3-hydroxy-2-methyl-4(1H)-pyridinones From Carbohydrate Precursors摘要:N-alkyl-3-hydroxy-2-methyl-4(1H)-pyridinohes can be synthesised in reasonable yield from either 1-(3-hydroxy-2-furanyl)ethanone or O-galactosylisomaltol. Since these carbohydrate precursors can be produced from the disaccharide, alpha-D-lactose, a simple and inexpensive transformation into pyridinones is possible.DOI:10.1080/00397919908086062
文献信息
-
Synthesis, characterization, and biological relevance of hydroxypyrone and hydroxypyridinone complexes of molybdenum作者:Sarah J Lord、Noah A Epstein、Robert L Paddock、Christopher M Vogels、Tracy L Hennigar、Michael J Zaworotko、Nicholas J Taylor、William R Driedzic、Tom L Broderick、Stephen A WestcottDOI:10.1139/v99-111日期:1999.7.1
We have prepared a number of complexes of the type cis-MoO2L2 where L represents a hydroxypyronato or hydroxypyridinonato ligand. Both the maltol (3-hydroxy-2-methyl-4-pyrone, Hma) and kojic acid (5-hydroxy-2-hydroxymethyl-4-pyrone, Hka) complexes, cis-MoO2(ma)2 (1) and cis-MoO2(ka)2 (2), have been characterized by X-ray diffraction studies. The pyrone ligands are bound to molybdenum in a cis bidentate fashion via the deprotonated hydroxyl groups and the ketone moieties. Crystals of 1 are orthorhombic, a = 12.107 (1), b = 8.6169 (8), c = 16.472 (1) Å, Z = 4, space group Pca21, and those of 2 are monoclinic, a = 8.4591 (5), b = 16.3453 (10), c = 10.2954 (7) Å, β = 103.0320 (10)°, Z = 4, space group P21/c. Hydroxypyridinone molybdenum complexes have been prepared for both maltol and kojic acid derivatives with the substituents Me, n-Pr, CH2Ph, Ph at the ring nitrogen. Crystals of the 3-hydroxy-2-methyl-1-phenyl-4-pyridinone (Hppp) derivative, MoO2(ppp)2 (9), are monoclinic, a = 10.9476 (6), b = 13.5353 (9), c = 17.4877 (10) Å, β = 93.465 (4)°, Z = 4, space group P21/n. Initial investigations into the effects molybdenum compounds have on diabetic hearts are presented. Both Na2MoO4 (used as a control) and 1 were effective in lowering blood glucose and free fatty acid levels. Diabetic rats treated with molybdate showed significant improvements in postischemic cardiac function.Key words: molybdenum, hydroxypyrones, hydroxypyridinones, heart function.
我们已经准备了一系列cis-MoO2L2类型的配合物,其中L代表羟基吡啶酮或羟基吡啶酮配体。麦芽酮(3-羟基-2-甲基-4-吡喃酮,Hma)和曲酸(5-羟基-2-羟甲基-4-吡喃酮,Hka)配合物,cis-MoO2(ma)2(1)和cis-MoO2(ka)2(2),已经通过X射线衍射研究进行了表征。吡喃酮配体以顺式双齿方式通过去质子羟基和酮基与钼结合。1的晶体为正交晶系,a = 12.107(1),b = 8.6169(8),c = 16.472(1)埃,Z = 4,空间群Pca21,而2的晶体为单斜晶系,a = 8.4591(5),b = 16.3453(10),c = 10.2954(7)埃,β = 103.0320(10)°,Z = 4,空间群P21/c。已经为麦芽酮和曲酸衍生物制备了羟基吡啶酮钼配合物,其在环氮原子处具有Me、n-Pr、CH2Ph、Ph等取代基。3-羟基-2-甲基-1-苯基-4-吡啶酮(Hppp)衍生物MoO2(ppp)2(9)的晶体为单斜晶系,a = 10.9476(6),b = 13.5353(9),c = 17.4877(10)埃,β = 93.465(4)°,Z = 4,空间群P21/n。首次研究了钼化合物对糖尿病心脏的影响。钼酸钠(用作对照)和1在降低血糖和游离脂肪酸水平方面均有效。接受钼酸盐治疗的糖尿病大鼠在缺血后心脏功能方面表现出显著改善。关键词:钼、羟基吡喃酮、羟基吡啶酮、心脏功能。 -
Process for producing pyrid-4-ones申请人:THE ROYAL FREE HOSPITAL SCHOOL OF MEDICINE公开号:EP0335745A1公开(公告)日:1989-10-04Substituted-3-hydroxy-pyrid-4-ones, derivatives thereof, and salts thereof are produced by reacting subsituted-3-hydroxypyr-4-one with ammonia or a non-sterically hindered primary amine usually in the presence of a solvent such as water. Also disclosed are novel derivatives of 2-ethyl-3-hydroxypyrid-4-one.
-
Synthetic methods and in vitro iron binding studies of the novel 1-alkyl-2-ethyl-3-hydroxypyrid-4-one iron chelators作者:G.J. Kontoghiorghes、L. Sheppard、J. BarrDOI:10.1016/s0020-1693(00)84951-8日期:1988.7Abstract Three novel iron chelators namely the 1-methyl-, 1-ethyl- and 1-propyl-2-ethyl-3-hydroxypyrid-4-ones were prepared in high yields from ethyl maltol and the related alkylamine in a one step reaction. These chelators formed 3 chelator:1 iron stable, coloured, neutral complexes at physiological pH and mobilise iron from transferrin, ferritin and haemosiderin. The rate of iron mobilisation from
-
VICINAL PRIMARY DIAMINES ASSOCIATED WITH METAL AND/OR FREE RADICAL CHELATION MOTIFS, AND ACTIVE AGAINST CARBONYL AND OXIDATIVE STRESS, AND USE THEREOF申请人:UNIVERSITE AMIENS PICARDIE JULES VERNE公开号:US20180201602A1公开(公告)日:2018-07-19The invention relates to compounds of Formula I: or the salts thereof, as well as the use thereof in the pharmaceutical, cosmetic or agrofood industry.该发明涉及公式I的化合物:或其盐,以及在制药、化妆品或农业食品工业中的使用。
-
Exploiting Natural Maltol for Synthesis of Novel Hydroxypyridone Derivatives as Promising Anti-Virulence Agents in Bactericides Discovery作者:Wan-Lin Xiao、Na Wang、Lin-Li Yang、Yu-Mei Feng、Pan-Long Chu、Jiao-Jiao Zhang、Shuai-Shuai Liu、Wu-Bin Shao、Xiang Zhou、Li-Wei Liu、Song YangDOI:10.1021/acs.jafc.3c00465日期:2023.5.3pathogenic bacteria. The antibacterial mechanism showed that it could considerably reduce various virulence factors (such as extracellular enzymes, biofilm, and T3SS effectors) and inhibit the expression of virulence factor-related genes. In addition, the control efficiency of compound B6 against rice bacterial leaf blight at 200 μg mL–1 was 46.15–49.15%, and their control efficiency was improved by approximately基于抑制细菌毒力因子的抗感染策略是开发新型抗菌药物以解决传统药物/农药杀菌活性引发的耐药性的重要方向。为了识别和获得更有效和多样化的毒力靶向分子,我们制备了一系列 3-hydroxy-2-methyl-1-pyridin-4-(1 H )-one衍生物并评估了它们的抗菌行为。化合物B 6表现出最高的生物活性,半数最大有效浓度 (EC 50 ) 值范围为 9m 10.03 至 30.16 μg mL –1对抗三种植物病原菌。抗菌机制表明它可以显着降低各种毒力因子(如细胞外酶、生物膜和T3SS效应子)并抑制毒力因子相关基因的表达。此外,复方B 6在200 μg·mL -1浓度下对水稻细菌性叶枯病的防效为46.15~49.15%,添加农药助剂后防效提高约12%。因此,开发了一类新的靶向细菌毒力因子的候选杀菌剂来控制植物细菌病害。
表征谱图
-
氢谱1HNMR
-
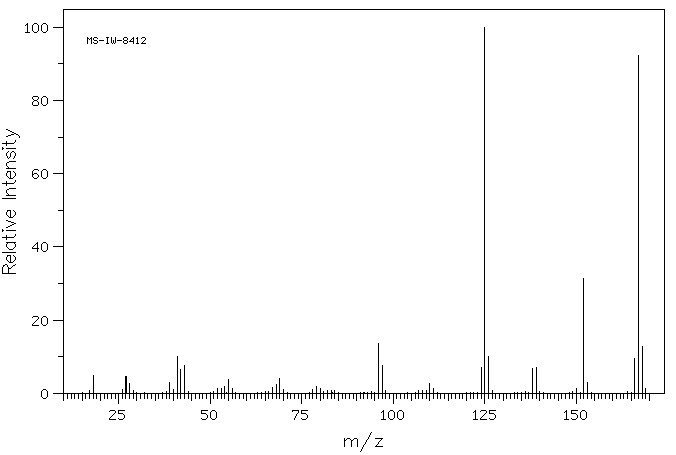
质谱MS
-
碳谱13CNMR
-
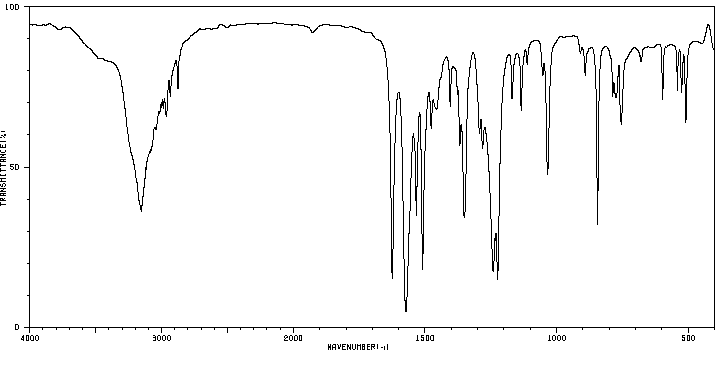
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息