methyl(1-phenylpropyl)sulfane | 28916-64-5
中文名称
——
中文别名
——
英文名称
methyl(1-phenylpropyl)sulfane
英文别名
1-methylsulfanylpropylbenzene
CAS
28916-64-5
化学式
C10H14S
mdl
——
分子量
166.287
InChiKey
HFFXYIAJXCLEPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-methylthio-3-phenyl-1-propanol —— C10H14OS 182.287 1-巯基-1-苯基丙烷 1-phenylpropane-1-thiol 76877-93-5 C9H12S 152.26 苯丙酮乙烷-1,2-二基二硫代缩醛 2-ethyl-2-phenyl-1,3-dithiolane 6008-82-8 C11H14S2 210.364
反应信息
-
作为反应物:参考文献:名称:Laarhoven,W.H. et al., Recueil des Travaux Chimiques des Pays-Bas, 1968, vol. 87, p. 1415 - 1420摘要:DOI:
-
作为产物:描述:参考文献:名称:铁催化卤化苄和二硫化物的交叉电偶合摘要:交叉亲电子偶联是有影响的反应,通常需要末端还原剂或光氧化还原条件。我们发现了一种铁催化反应,在没有末端还原剂的情况下,在光氧化还原条件下,苄基卤与二硫化物偶联生成硫醚产物。所公开的平台无需硫引起的催化剂中毒或使用外源碱,支持广泛的范围并避免不期望的消除途径。我们将开发的化学方法应用于二硫键生物共轭、药物合成、克级合成和产品衍生化的新模式。最后,我们进行了机械实验,以更好地了解两种亲电子试剂之间的立体消融反应。二硫化物和苄基硫醚对于生物和制药应用至关重要,但与醚类和氨基对应物相比,其研究仍然严重不足。因此,我们预计这个铁催化平台和下游应用会引起更大的科学界的兴趣。DOI:10.1021/jacs.3c13984
文献信息
-
Formation of Cyclopropanes via Activation of (γ-Methoxy)alkyl Gold(I) Complexes with Lewis Acids作者:Nana Kim、Ross A. WidenhoeferDOI:10.1021/acs.organomet.0c00324日期:2020.9.14Treatment of the gold 3-methoxy-3-phenylpropyl complex (P)AuCH2CH2CH(OMe)Ph [P = P(t-Bu)2o-biphenyl] with AlCl3 at −78 °C led to the immediate (≤5 min) formation of a 4:1 mixture of phenylcyclopropane and (1-methoxypropyl)benzene in 86 ± 5% combined yield. Lewis acid activation of the stereochemically pure isotopomer erythro-(P)AuCH2CHDCH(OMe)Ph led to the formation of cis-2-deuterio-1-phenylcyclopropane在−78°C下用AlCl 3处理3-甲氧基-3-苯基丙基金(P)AuCH 2 CH 2 CH(OMe)Ph [ P = P(t -Bu)2 o-联苯]金导致立即(≤5分钟)形成苯基环丙烷和(1-甲氧基丙基)苯的4:1混合物,合并产率为86±5%。立体化学纯的异构体赤型-(P)AuCH 2 CHDCH(OMe)Ph的路易斯酸活化导致顺式形成-2-氘代-1-苯基环丙烷为单一立体异构体,产率为84±5%,这表明环丙烷化是随着γ-立体中心的反转而发生的。同样,立体化学纯的环己基金配合物顺式-(P)Au CHCH 2 CH(OMe)CH 2 CH 2 CH 2在-78°C下电离形成双环[3.1.0]己烷,产率为82%±5%,验证了涉及α-立体中心反转的环丙烷化低能途径。综上所述,这些观察结果与环丙烷形成机理一致,该机理涉及Cγ离去基团和Cα(L)Au +片段通过W形过渡态向后位移。
-
The Lithium Diisopropylamide-induced Fragmentation of 1,3-Dithiolane Derivatives of Several Ketones Having α-Hydrogen作者:Hideyuki Ikehira、Shigeo Tanimoto、Tatsuo OidaDOI:10.1246/bcsj.56.2537日期:1983.8The reaction of 1,3-dithiolane derivatives of ketones having α-hydrogen with lithium diisopropylamide results in fragmentation to the corresponding thioketone followed by further conversion in a few steps to the other intermediate species which, on trapping with alkyl halide, leads to a vinylic sulfide and/or a sulfide bearing a secondary alkyl group.
-
The Reaction of Sulfenyl Chlorides with Thioethers. I. The Scope of the Reactions作者:Michinori Oki、Keiji KobayashiDOI:10.1246/bcsj.43.1223日期:1970.4producing a rather stable carbonium ion, the thioether gives the chloride and an olefin, which are derived from the cation, the latter reacting also with the sulfenyl chloride to give adducts. There is another path to afford alkyl chloride; that is, an SN2-type reaction takes place when the alkyl part gives a less stable carbonium ion. The third and fourth products are α-chloro sulfide and α,β-unsaturated
-
AQUEOUS INK COMPOSITION FOR INK-JET RECORDING, AND IMAGE FORMATION METHOD申请人:FUJIFILM Corporation公开号:EP3521385A1公开(公告)日:2019-08-07Provided are an ink jet recording aqueous ink composition including: a coloring material; water; an organic solvent d-1 which is represented by Formula 1 or 2 and which has a ClogP value of 0.5 to 3.5; and a resin particle including a resin containing 1 mass% to 20 mass% of a monomer unit c-1 having a ClogP value more than the ClogP value of the organic solvent d-1 and having an anionic group, with respect to a total mass of the resin, in which a content of the organic solvent d-1 is 0.5 mass% to 10 mass% with respect to a total mass of the ink jet recording aqueous ink composition, and an image forming method.
-
Banthorpe,D.V. et al., Journal of the Chemical Society, 1960, p. 4054 - 4087作者:Banthorpe,D.V. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
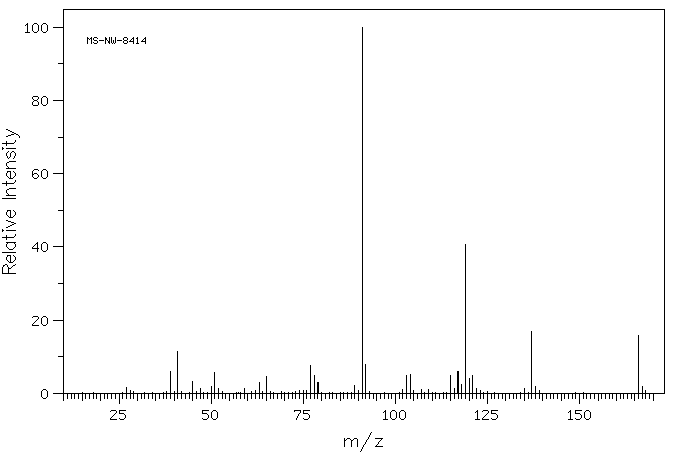
质谱MS
-
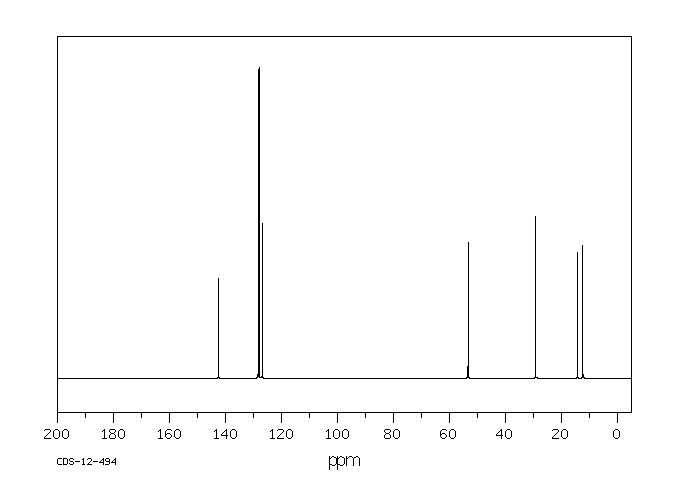
碳谱13CNMR
-
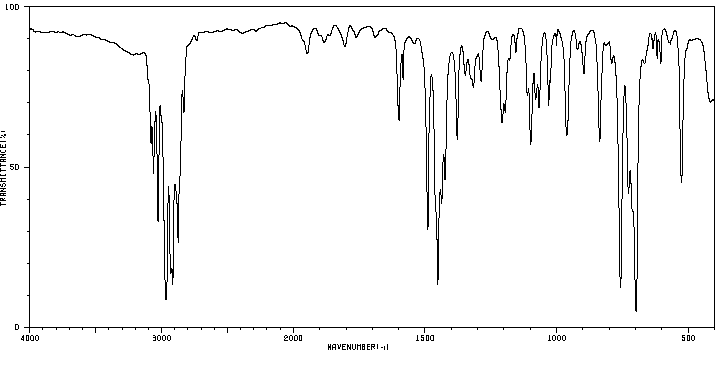
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫