N,N-DIMETHYL-4,4'-AZODIANILINE | 539-17-3
中文名称
——
中文别名
——
英文名称
N,N-DIMETHYL-4,4'-AZODIANILINE
英文别名
4-amino-4'-dimethylaminoazobenzene;(E)-4-((4-aminophenyl)diazenyl)-N,N-dimethylaniline;(E)-4-[(4-aminophenyl)diazenyl]-N,N-dimethylaniline;(E)-4'-amino-4-(dimethylamino)azobenzene;N,N-dimethyl-4,4’-azodianiline;4-amino-4'-(dimethylamino)azobenzene
CAS
539-17-3;70735-04-5
化学式
C14H16N4
mdl
——
分子量
240.308
InChiKey
BVRIUXYMUSKBHG-WUKNDPDISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:190-195 °C (dec.)
-
沸点:373.02°C (rough estimate)
-
密度:1.0236 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):3.75
-
重原子数:18.0
-
可旋转键数:3.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:53.98
-
氢给体数:1.0
-
氢受体数:4.0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2927000090
-
RTECS号:BX5020000
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-N-(4-{[4-(dimethylamino)phenyl]diazenyl}phenyl)acetamide —— C16H18N4O 282.345 —— N,N-dimethyl-4-[(E)-(4-nitrophenyl)diazenyl]aniline 55252-43-2 C14H14N4O2 270.291 N,N-二甲基-对苯二胺 4-amino-N,N-dimethylaniline 99-98-9 C8H12N2 136.197 N,N-二甲基-4-亚硝基苯胺 p-dimethylaminonitrosobenzene 138-89-6 C8H10N2O 150.18 N,N-二甲基苯胺 N,N-dimethyl-aniline 86362-18-7 C8H11N 121.182 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(N,N-DIMETHYLAMINO)AZOBENZENE-4'-ISOTHIOCYANATE 7612-98-8 C15H14N4S 282.369 —— (E)-N-(4-{[4-(dimethylamino)phenyl]diazenyl}phenyl)acetamide —— C16H18N4O 282.345
反应信息
-
作为反应物:描述:N,N-DIMETHYL-4,4'-AZODIANILINE 以68%的产率得到参考文献:名称:Coe Benjamin J., Foulon Jean-Dominic, Hamor Thomas A., Jones Christopher +, J. Chem. Soc. Dalton Trans., (1994) N 23, S 3427-3439摘要:DOI:
-
作为产物:参考文献:名称:ISKIERKO, JERZY;PYRA, EDMUND摘要:DOI:
文献信息
-
[EN] PROCESS FOR THE PREPARATION OF ASYMMETRICAL BIS(THIOSEMICARBAZONES)<br/>[FR] PROCÉDÉ DE PRÉPARATION DE BIS(THIOSEMICARBAZONES) ASYMÉTRIQUES申请人:UNIV MELBOURNE公开号:WO2010066010A1公开(公告)日:2010-06-17The present invention relates to a method of making asymmetrical bis(thiosemicarbazones), compounds useful as synthetic intermediates in the method, new bis(thiosemicarbazones) that can be readily accessed by use of the method and methods of treatment and imaging utilising some of the new bis(thiosemicarbazones).
-
Bidentate and Tetradentate β‐Aminovinyl Trifluoromethylated Ketones and Their Copper(II) Complexes: Synthesis, Characterization and Redox Chemistry作者:Nicolas Chopin、Maurice Médebielle、Guillaume PiletDOI:10.1002/ejic.201101385日期:2012.3polyfunctional bidentate and tetradentate trifluoromethylated enaminone ligands that bear redox-active and photosensitive moieties were synthesized in moderate to good yields and their coordination chemistry with copper(II) was examined. The crystal structures revealed the formation of mononuclear CuII complexes with all ligands, where the metal ion is located in almost perfect to distorted square planar environments
-
MULTI-TARGETING AGENTS FOR ALZHEIMER'S DISEASE THERAPY申请人:The Regents of the University of California公开号:US20200171016A1公开(公告)日:2020-06-04The disclosure provides for multi-targeting agents that assist in the targeted removal of amyloid beta plaques from the brain and surrounding vasculature of subject's with Alzheimer's disease, and methods of treatment thereof.该披露提供了一种多靶向药剂,可帮助定向清除患有阿尔茨海默病的受试者大脑和周围血管系统中的淀粉样蛋白斑块,并提供了相应的治疗方法。
-
Azo compounds reducing formation and toxicity of amyloid beta aggregation intermediates申请人:Mdc Max-Delbrück-Centrum Für Molekulare Medizin Berlin - Buch公开号:EP2368558A1公开(公告)日:2011-09-28The present invention relates to compounds suitable as modulators of protein misfolding and/or protein aggregation. The compounds are particularly suitable as inhibitors of amyloid aggregate formation and/or modulators of amyloid surface properties, and/or as activators of degradation or reduction of amyloid aggregates.本发明涉及适用作蛋白质错误折叠和/或蛋白质聚集调节剂的化合物。这些化合物特别适用作淀粉样聚集物形成的抑制剂和/或淀粉样表面特性的调节剂,和/或作为淀粉样聚集物的降解或减少的激活剂。
-
Azo-based phenylthiophene Schiff bases: Syntheses, crystal structures and optical properties作者:Afef Shili、Awatef Ayadi、Said Taboukhat、Nabil Zouari、Bouchta Sahraoui、Abdelkrim El-GhayouryDOI:10.1016/j.molstruc.2020.128933日期:2020.12yield. These two compounds were subjected to the following characterization techniques: single crystal X-ray diffraction, elemental analysis, infrared, mass, 1H NMR, 13C NMR spectroscopy and thermogravimetric analysis. The Schiff bases A and B belong to the monoclinic P21/c space group. Their crystal structures are stabilized by intra and intermolecular hydrogen bonds, as well as by weak π−π stacking.摘要 基于两种新的有机材料供体受体系统,即 N,N-二甲基-4-((E)-(4-((E)-((5-phenylthiophen-2-yl)methylene)amino)phenyl)diazenyl)成功合成了苯胺A和(E)-4-((E)-(4-硝基苯基)二氮烯基)-N-((5-苯基噻吩-2-基)亚甲基)苯胺B。两种化合物(A 和 B)都是通过 N, N-Dimethyl-4,4'-azodianiline 或 4-(4-nitrophenylazo)aniline 与 5-phenylthiophene-2- 甲醛之间的缩合反应合成的,77% 和 83 % 屈服。对这两种化合物进行以下表征技术:单晶 X 射线衍射、元素分析、红外、质谱、1H NMR、13C NMR 光谱和热重分析。席夫碱 A 和 B 属于单斜 P21/c 空间群。它们的晶体结构通过分子内和分子间氢键稳定,以及弱 π−π
表征谱图
-
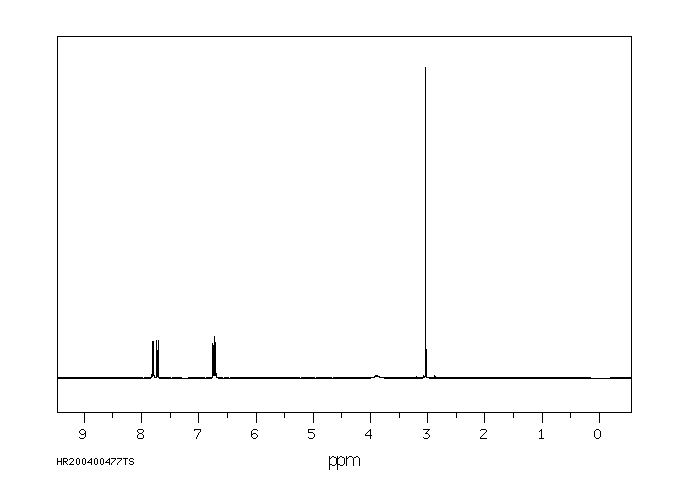
氢谱1HNMR
-
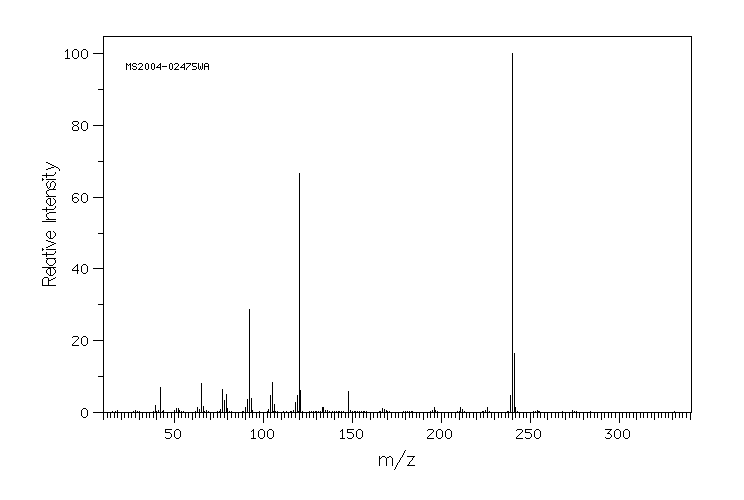
质谱MS
-
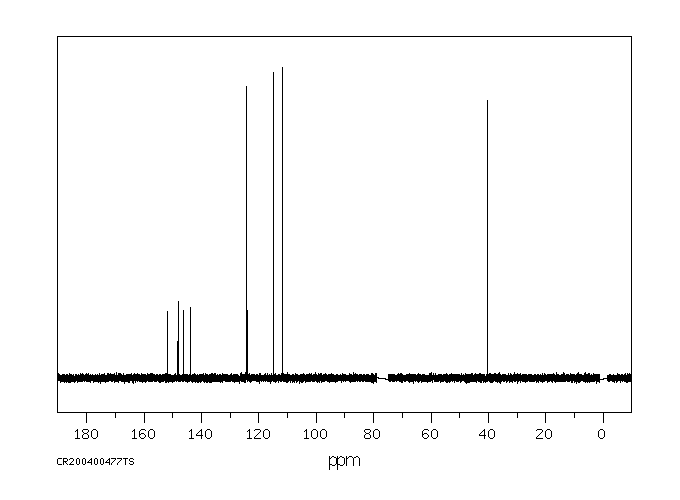
碳谱13CNMR
-
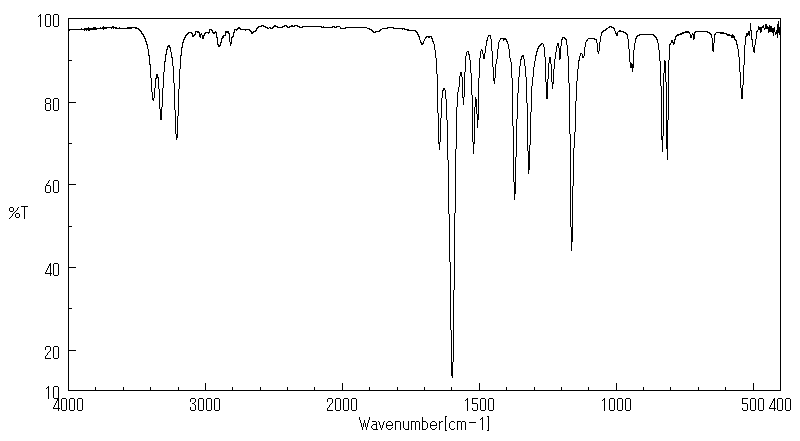
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黑洞猝灭剂-2,BHQ-2ACID
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S,24S)-
颜料橙61
阿利新黄GXS
阳离子红X-GTL
阳离子红5BL
阳离子橙RN
阳离子橙GLH
间甲基红
镨(3+)丙烯酰酸酯
镍酸酯(1-),[3-羟基-4-[(4-甲基-3-硫代苯基)偶氮]-2-萘羧酸根(3-)]-,氢
锂3-({4-[(4-羟基苯基)偶氮]-5-甲氧基-2-甲基苯基}偶氮)苯磺酸酯
钴,[二[m-[[1,2-二苯基-1,2-乙二酮1,2-二(肟酸根-kO)](2-)]]四氟二硼酸根(2-)-kN1,kN1',k2,kN2']-,(SP-4-1)-
钠5-氯-2-羟基-3-[(2-羟基-4-{[(4-甲基苯基)磺酰基]氧基}苯基)偶氮]苯磺酸酯
钠5-[[3-[[5-[[4-[[[4-[(4,5-二氢-3-甲基-5-氧代-1H-吡唑-4-基)偶氮]苯基]氨基]羰基]苯基]偶氮]-2,4-二羟基苯基]偶氮]-4-羟基苯基]偶氮]水杨酸盐
钠4-[(4-氨基苯基)偶氮]苯甲酸酯
钠4-[(4-{[4-(二乙基氨基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-[(4-{[2-羟基-5-(2-甲基-2-丙基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-({3-甲氧基-4-[(4-甲氧基苯基)偶氮]苯基}偶氮)苯磺酸酯
钠3-[4-(2-羟基-5-甲基-苯基)偶氮苯基]偶氮苯磺酸酯
钠3-({5-甲氧基-4-[(4-甲氧基苯基)偶氮]-2-甲基苯基}偶氮)苯磺酸酯
钠3-({4-[(4-羟基-2-甲基苯基)偶氮]-3-甲氧基苯基}偶氮)苯磺酸酯
金莲橙O
重氮基烯,苯基[4-(三氟甲基)苯基]-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(Z)-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(E)-
重氮基烯,[4-[(2-乙基己基)氧代]-2,5-二甲基苯基](4-硝基苯基)-
重氮基烯,1,2-二(4-丙氧基苯基)-,(1E)-
重氮基烯,(2-氯苯基)苯基-
酸性金黄G
酸性棕S-BL
酸性媒染棕
酸性媒介棕6
酸性媒介棕48
酸性媒介棕4
酸性媒介棕24
邻氨基偶氮甲苯
达布氨乙基甲硫基磺酸盐
赛甲氧星
茴香酸盐己基
茜素黄 R 钠盐
苯重氮化,2-甲氧基-5-甲基-4-[(4-甲基-2-硝基苯基)偶氮]-,氯化
苯酰胺,4-[4-(2,3-二氢-1,4-苯并二噁英-6-基)-5-(2-吡啶基)-1H-咪唑-2-基]-
苯酚,4-(1,1-二甲基乙基)-2-(苯偶氮基)-
苯酚,2-甲氧基-4-[(4-硝基苯基)偶氮]-
苯胺棕
苯胺,4-[(4-氯-2-硝基苯基)偶氮]-
苯磺酸,3,3-6-(4-吗啉基)-1,3,5-三嗪-2,4-二基二亚氨基2-(乙酰基氨基)-4,1-亚苯基偶氮二-,盐二钠
苯磺酸,2-[(4-氨基-2-羟基苯基)偶氮]-
苯甲酸,5-[[4-[(乙酰基氨基)磺酰]苯基]偶氮]-2-[[3-(三氟甲基)苯基]氨基]-(9CI)