1-(三甲硅基)-1H-1,2,4-三唑 | 18293-54-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:74 °C12 mm Hg(lit.)
-
密度:0.989 g/mL at 25 °C(lit.)
-
闪点:8°C
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):0.74
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:30.7
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F,Xi
-
安全说明:S16,S26
-
危险类别码:R11
-
WGK Germany:3
-
危险品运输编号:UN 1993 3/PG 2
-
海关编码:2933990090
-
包装等级:II
-
危险类别:3
-
危险性防范说明:P210,P240,P242,P243,P261,P264,P271,P280,P303+P361+P353,P304+P340,P305+P351+P338,P312,P363,P370+P378,P403+P233,P501
-
危险性描述:H225,H315,H319,H335
-
储存条件:常温下应密闭避光、保持通风和干燥,并在惰性气体保护下存储。
SDS
反应信息
-
作为反应物:描述:1-(三甲硅基)-1H-1,2,4-三唑 在 硫酰氟 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 作用下, 以 二氯甲烷 为溶剂, -69.0~20.0 ℃ 、93.0 kPa 条件下, 反应 12.0h, 以87%的产率得到1,2,4-triazole-1-sulfonyl fluoride参考文献:名称:[EN] SULFONYLDIAZOLES AND N-(FLUOROSULFONYL)AZOLES, AND METHODS OF MAKING THE SAME
[FR] SULFONYLDIAZOLES ET N-(FLUOROSULFONYL)AZOLES, ET LEURS PROCÉDÉS DE PRÉPARATION摘要:公开号:WO2020210174A4 -
作为产物:描述:参考文献:名称:[EN] PROCESS AND INTERMEDIATE OF LETROZOLE
[FR] PROCÉDÉ ET PRODUIT INTERMÉDIAIRE DU LÉTROZOLE摘要:本发明涉及一种利用新的I式中间体制备来曲唑的方法。公开号:WO2009078036A2 -
作为试剂:参考文献:名称:[EN] PROCESS AND INTERMEDIATE OF LETROZOLE
[FR] PROCÉDÉ ET PRODUIT INTERMÉDIAIRE DU LÉTROZOLE摘要:本发明涉及一种利用新的I式中间体制备来曲唑的方法。公开号:WO2009078036A2
文献信息
-
<i>N</i>-(<i>tert</i>-Butyldimethylsilyl)imidazole and related heterocycles: <sup>13</sup>C nuclear magnetic resonance study and reaction with dimethylsulfoxide作者:Alexander F. Janzen、Gerald N. Lypka、Roderick E. WasylishenDOI:10.1139/v80-010日期:1980.1.1
N-tert-Butyldimethylsilyl derivatives of imidazole, 2-methylimidazole, 4-methylimidazole, benzimidazole, pyrazole, 1,2,4-triazole, and benzotriazole were prepared from tert-butyldimethylsilyl chloride and the corresponding heterocyclic compound. The products were identified by carbon and proton nmr, mass spectrometry, and elemental analysis. The carbon nmr spectra confirmed the absence of intermolecular silyl exchange at ambient temperature. Silyl exchange did occur at elevated temperatures, 130–160 °C.Reaction of N-tert-butyldimethylsilyl or N-trimethylsilyl heterocycles with dimethylsulfoxide gave N-(methylthio)methyl derivatives of imidazole, 2-methylimidazole, 4-methylimidazole, benzimidazole, pyrazole, and 1,2,4-triazole. The products were characterized by carbon and proton nmr, mass spectrometry, and elemental analysis. A mechanism involving a Pummerer rearrangement is proposed to account for the results.
-
Nucleophilic activation of hydrogen peroxide. On the formation and the reactivity of 1H-1,2,4-triazoleperoxycarboxylic acid and O-p-nitrophenylmonoperoxycarbonic acid †作者:Carsten Bender、Hans-Dieter BrauerDOI:10.1039/a903852c日期:——Measurements of the infrared phosphorescence of singlet molecular oxygen (1O2) at 1270 nm have been employed to demonstrate the formation of 1O2 in the system N,Nâ²-carbonyldi-1,2,4-triazole (CDT)âhydrogen peroxide and in the system 1H-1,2,4-triazolecarboxylic acid p-nitrophenyl ester (TCNP)âhydrogen peroxide in tetrahydrofuran. At hydrogen peroxide concentrations of [H2O2] â¥Â 2 [CDT] or â¥Â 2 [TCNP] one molecule of 1O2 is generated per molecule of CDT and TCNP, respectively. In both systems a very reactive peroxy-intermediate is formed. In the system CDTâH2O2 the 1H-1,2,4-triazoleperoxycarboxylic acid (1) is generated, in TCNPâH2O2 the p-nitrophenylmonoperoxycarbonic acid (4). For the epoxidation of cyclohexene in THF by 1 a rate constant k15 â3 Ã 100 dm3 molâ1 sâ1 can be estimated. For 4 the corresponding rate constant was found to be k20 â 6 Ã 10â2 dm3 molâ1 sâ1. The Arrhenius parameters of the formation of 1 and 4, respectively, and in addition of their consecutive reactions with hydrogen peroxide were determined. The results are consistent with the assumption that the nucleophilic displacements by hydrogen peroxide at the carbonyl carbon atom are additionâelimination processes, which take the form of a BAC2-mechanism.在四氢呋喃中,通过测量单线态分子氧(1O2)在1270 nm处的红外磷光,证明了在N,N'-羰基二-1,2,4-三唑(CDT)–过氧化氢体系和1H-1,2,4-三唑羧酸对硝基苯酯(TCNP)–过氧化氢体系中1O2的生成。当过氧化氢浓度[ ]≥2[CDT]或≥2[TCNP]时,CDT和TCNP分子各自生成一个1O2分子。在这两个体系中,都形成了一个高度活性的过氧中间体。在CDT–H2O2体系中生成1H-1,2,4-三唑过氧羧酸(1),在TCNP– 体系中生成对硝基苯单过氧碳酸(4)。对于1在THF中环己烯环氧化反应的速率常数k15≈3×100 dm3 mol−1 s−1可以估算出来。对于4,相应的速率常数被发现为k20≈6×10−2 dm3 mol−1 s−1。分别确定了1和4的形成以及它们与过氧化氢的连续反应的阿伦尼乌斯参数。结果与假设一致,即过氧化氢在羰基碳原子上的亲核取代是加成-消除过程,表现为BAC2机制。
-
Nucleotide compound, nucleotide block oligonucleotide, and method for producing them申请人:Toagosei Company, Ltd.公开号:US06380378B1公开(公告)日:2002-04-30Novel nucleotide compounds represented by the formula (I) wherein R1 represents a protective group or a PEG bearing organic group; R2, R2′, R2″, R3, R3′ and R3″ each represents a hydrogen atom or an alkyl, cycloalkyl, aryl or aralkyl group which may contain a hetero-atom; B1, B2, B3 and B4 each represents a base, if necessary, protected by a protective group common in nucleotide chemistry or by a PEG bearing organic group; X, X′ and X″ each represents an oxygen atom or a sulfur atom; Y represents an azolyl group, a mono- or di-alkylamino group or a saturated nitrogenous heterocyclic ring; A1, A2, A3 and A4 each represents a hydrogen atom, a hydroxyl group, an alkoxy group or a trialkylsilyloxy group; and m and n each represents 0 or an integer of 1 to 100. The nucleotide of the formula (I) can be used as it is in a reaction mixture for further reaction with 3′-O- and 5′-O-unprotected nucleoside or nucleotide of the formula (II) to yield a nucleotide block or oligonucleotide, and thus is useful as in situ DNA synthesis reagents.根据公式(I)表示的新型核苷酸化合物: 其中R1代表保护基团或携带有机基团的聚乙二醇;R2、R2′、R2″、R3、R3′和R3″每个代表氢原子或可能含有杂原子的烷基、环烷基、芳基或芳烷基团;B1、B2、B3和B4每个代表一个碱基,如有必要,由核苷酸化学中常见的保护基团或携带有机基团的聚乙二醇保护;X、X′和X″每个代表氧原子或硫原子;Y代表唑基团、单烷基或二烷基氨基团或饱和氮杂环;A1、A2、A3和A4每个代表氢原子、羟基、烷氧基或三烷基硅氧基;m和n每个代表0或1到100的整数。公式(I)的核苷酸可以以其本身的形式用于反应混合物中,与3′-O-和5′-O-未保护的公式(II)的核苷或核苷酸进一步反应,产生核苷酸块或寡核苷酸,因此作为原位DNA合成试剂非常有用。
-
A Novel, Simple Cyclocondensation Reaction Towards Glycosyl Triazines作者:David Deniaud、Vincent Kikelj、Karine Julienne、Pascal Janvier、Jean-Claude MeslinDOI:10.1055/s-0028-1083156日期:——Sugars bearing an isothiocyanate moiety at C-1 react with diazadienium iodide to afford glycosyl triazines that represent, through an easy cyclocondensation reaction step, a flexible entry to different nucleoside analogues. We herein demonstrate that this [4+2] cycloaddition reaction occurs with total regiocontrol and good yields. Subsequent transformation of the thiocarbonyl into a carbonyl, and nucleophilic substitution of the methylsulfanyl group by ammonia, yields the 5-azacytidine analogues. All compounds were fully characterised by IR, HRMS, and ¹³C and ¹H NMR (COSY, HMBC and HMQC).
-
Energetic polyazole polynitrobenzenes and their coordination complexes作者:Zhuo Zeng、Yong Guo、Brendan Twamley、Jean’ne M. ShreeveDOI:10.1039/b915090k日期:——New energetic polyazole polynitrobenzenes exhibit high heats of formation and thermal stabilities, and react as useful ligands with silver dinitramide to give an energetic coordination complex.
表征谱图
-
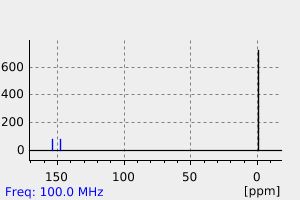
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息