4-氯庚烷 | 998-95-8
中文名称
4-氯庚烷
中文别名
——
英文名称
4-chloroheptane
英文别名
4-Chlor-heptan
CAS
998-95-8
化学式
C7H15Cl
mdl
——
分子量
134.649
InChiKey
MGSGWAXIEMEWCQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-69.5°C (estimate)
-
沸点:152.39°C (estimate)
-
密度:0.8710
-
保留指数:941;949;959;900;900
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903199000
SDS
反应信息
-
作为反应物:参考文献:名称:Pritzkow; Mahler, Journal fur praktische Chemie (Leipzig 1954), 1959, vol. <4>8, p. 314,316,321摘要:DOI:
-
作为产物:参考文献:名称:聚氯乙烯热脱氯化氢中的自催化机理摘要:聚氯乙烯(PVC)热脱氯化氢过程中的自催化被证明是一种自由基过程,可将聚合物的普通单体单元转化为热稳定性低的氯代烯丙基结构。在脱氯化氢的第一阶段,通过非自由基途径产生共轭多烯序列。它们与HCl反应生成阳离子单自由基和/或激发的阳离子双自由基。这些物种中的一个或两个,或由它们形成的其他自由基,可以提取亚甲基氢,以产生新的也以碳为中心的自由基。它们通过氯原子的β断裂转变成氯代烯丙基链段,以通常的(非自由基)方式开始新的多烯的生长。在固态PVC中,在180°C下,自由基清除剂(受阻酚,(三苯基甲烷和金属汞),但同时引入全反式β-胡萝卜素(一种PVC多烯序列的模型)时,由于HCl浓度的增加而大大增强了该效果。当它们处于自催化条件下时,其他模型化合物所得到的产物显然是自由基中间体从氢中提取出来的产物。DOI:10.1021/ma0352835
文献信息
-
Method for producing organic compounds by substituting halogen atoms申请人:MITSUI CHEMICALS, INC.公开号:EP1486479A1公开(公告)日:2004-12-15The invention pertains to a method in which a halogen atom of an organic compound is replaced with a group derived from a nucleophilic agent, at high yield and high efficiency, by the following method which includes a step of reacting the nucleophilic agent with an organic material having a halogen atom bonded to a carbon atom having four σ bonds, more specifically: a method for producing an organic compound having Q, the method including a step of reacting a compound represented by general formula (2) with an organic starting material having at least one halogen atom bonded to a carbon atom having four σ bonds so as to replace the halogen atom in the organic starting material with Q: MQa (2) (wherein M represents an alkali metal atom, an alkali earth metal atom, or a rare earth metal atom; Q represents a moiety of an inorganic acid or an active hydrogen compound derived by eliminating a proton, wherein Q is a halogen atom different from the halogen atom in the organic starting material having the halogen atom bonded to the carbon atom having the four σ bonds; and a represents an integer of 1 to 3) in the presence of a compound represented by general formula (1) (wherein Z- represents an anion derived by eliminating a proton from an inorganic acid or an active hydrogen compound; R2 is the same or different; R2 each independently represent a C1-C10 hydrocarbon group or two R2 on the same nitrogen atom may be bonded with each other to form a ring with the nitrogen atom).这项发明涉及一种方法,其中有机化合物中的卤素原子被来自亲核试剂的基团取代,且产率高效率高,通过以下方法实现,包括以下步骤:将亲核试剂与具有与碳原子形成四个σ键的卤素原子相结合的有机材料反应的步骤,更具体地说:一种用于生产具有Q的有机化合物的方法,包括以下步骤:将由通式(2)表示的化合物与至少一个卤素原子与碳原子形成四个σ键的有机起始材料反应,以将有机起始材料中的卤素原子替换为Q: MQa (2) (其中M代表碱金属原子、碱土金属原子或稀土金属原子;Q代表由消除质子衍生的无机酸或活性氢化合物的基团,其中Q是不同于有机起始材料中卤素原子的卤素原子,该卤素原子与具有四个σ键的碳原子相结合;a表示1到3的整数),在通式(1)表示的化合物的存在下 (其中Z-代表由无机酸或活性氢化合物中消除质子衍生的阴离子;R2相同或不同;R2各自独立地表示C1-C10烃基,或者两个R2在同一氮原子上可能与彼此结合形成与氮原子的环)。
-
Symmetric and asymmetric proton transfer from heptane and octane radical cations to heptane molecules in γ-irradiated n-C<sub>7</sub>H<sub>16</sub>–n-C<sub>8</sub>H<sub>18</sub>–2-C<sub>6</sub>H<sub>13</sub>Cl crystals: structural disorder in mixed alkane crystals作者:Adelheid Demeyer、Jan CeulemansDOI:10.1039/b206335m日期:——A study has been made of the isomeric composition of secondary chloroheptanes formed upon γ-irradiation at 77 K and subsequent melting of heptane containing 1 mol% 2-chlorohexane and various concentrations of octane. It is observed that the relative importance of 2-chloroheptane increases as a result of the presence of octane in the crystallites. This increase is attributed to selective proton transfer from ground state heptane radical cations to penultimate CâH bonds in heptane molecules. The proton transfer is induced by (partial) dislocation of heptane molecules adjacent to octane solute molecules, which brings the penultimate heptane CâH bonds into close contact with planar chain-end CâH bonds in heptane radical cations. Proton transfer from extended all-trans octane radical cations (on which the positive hole temporarily resides) to heptane molecules may also contribute to the observed effect. The results have important implications with respect to the molecular packing in binary n-alkane crystals. In binary n-alkane crystals, in which the shorter component is predominant, molecules of the longer component cannot be fully accommodated in one molecular layer of the crystal, even with a chain length mismatch of only one methylene unit; instead (at least partial) dislocation of adjacent molecules of the shorter component takes place. Such dislocations do not extend indefinitely over the crystal, however, and crystal order is restored by squeezing, deformation and changes in conformation of the appropriate molecules, including molecules of the shorter component.对含有 1 mol% 2-氯己烷和不同浓度辛烷的庚烷在 77 K 下经过δ-辐照并随后熔化后形成的仲氯庚烷的异构体组成进行了研究。据观察,由于晶体中存在辛烷,2-氯庚烷的相对重要性增加。这种增加归因于质子从基态庚烷自由基阳离子向庚烷分子中倒数第二个 CâH 键的选择性转移。质子转移是由邻近辛烷溶质分子的庚烷分子(部分)错位引起的,错位使倒数第二位庚烷 CâH 键与庚烷自由基阳离子中的平面链端 CâH 键紧密接触。从扩展的全反式辛烷自由基阳离子(正空穴暂时位于其上)向庚烷分子的质子转移也可能有助于观察到的效果。这些结果对于二元正烷烃晶体中的分子堆积具有重要意义。在二元正烷烃晶体中,短组分占主导地位,即使只有一个亚甲基单元的链长不匹配,长组分的分子也不能完全容纳在晶体的一个分子层中;相反,相邻的短组分分子会发生(至少部分)错位。然而,这种错位不会无限地延伸到整个晶体,晶体秩序会通过挤压、变形和适当分子(包括较短组分的分子)的构象变化而得到恢复。
-
Efficient TBAI-CS<sub>2</sub> Promoted Synthesis of Substituted Hydrazinecarbodithiolates作者:Nitin SrivastavaDOI:10.1080/00304948.2022.2111171日期:2022.11.2Published in Organic Preparations and Procedures International: The New Journal for Organic Synthesis (Vol. 54, No. 6, 2022)发表于国际有机制剂和程序:有机合成新期刊(印刷前,2022 年)
-
Bile acid analogs as FXR/TGR5 agonists and methods of use thereof申请人:Enanta Pharmaceuticals, Inc.公开号:US10266560B2公开(公告)日:2019-04-23The present invention relates to compounds of Formula (I), and pharmaceutically acceptable salts thereof, where R1, R2, R3, R4, R5, R6, R7, R8 and m are as defined herein, pharmaceutical compositions comprising these compounds and methods of use of these compounds for treating a TGR5 mediated disease or condition.本发明涉及式(I)化合物、 及其药学上可接受的盐,其中 R1、R2、R3、R4、R5、R6、R7、R8 和 m 如本文所定义;包含这些化合物的药物组合物;以及使用这些化合物治疗 TGR5 介导的疾病或病症的方法。
-
Method for producing organic compound by substituting halogen atoms申请人:Mitsui Chemicals, Inc.公开号:US20040256743A1公开(公告)日:2004-12-23A method for producing an organic compound having Q, the method including a step of reacting a compound represented by general formula (2) with an organic starting material having at least one halogen atom bonded to a carbon atom having four &sgr; bonds so as to replace the halogen atom in the organic starting material with Q: MQ a (2) wherein M, Q and a are defined in the presence of a compound represented by general formula (1) 1 wherein Z − and Rs are also defined.一种生产具有 Q 的有机化合物的方法,该方法包括以下步骤:将通式(2)代表的化合物与有机起始材料反应,该有机起始材料具有至少一个与具有四个&sgr;键的碳原子成键的卤原子,从而用 Q 取代有机起始材料中的卤原子: MQ a (2) 其中 M、Q 和 a 是在通式(1)所代表的化合物存在时定义的 1 其中 Z - 和 Rs 也已定义。
表征谱图
-
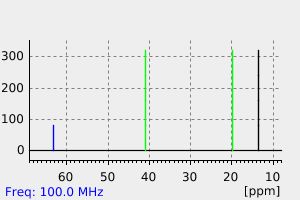
氢谱1HNMR
-
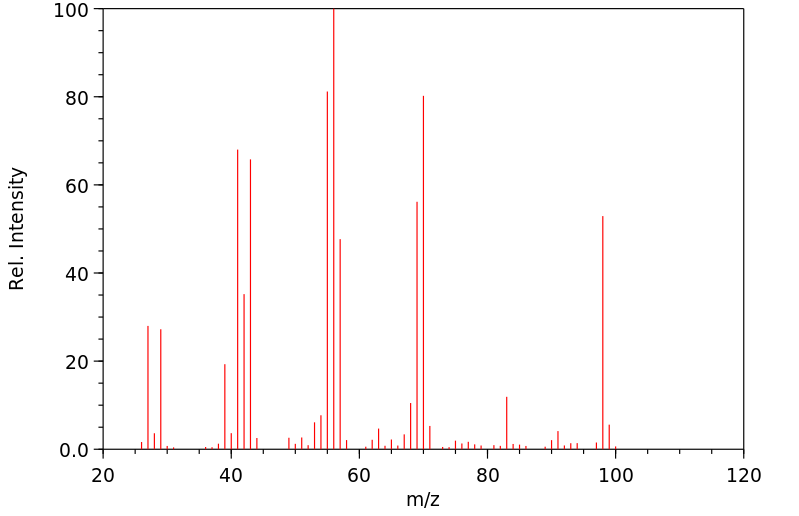
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷