5,6-二氢-4H-1,3-二噻英-2-硫酮 | 1748-15-8
中文名称
5,6-二氢-4H-1,3-二噻英-2-硫酮
中文别名
——
英文名称
1,3-dithiane-2-thione
英文别名
——
CAS
1748-15-8
化学式
C4H6S3
mdl
MFCD08692722
分子量
150.29
InChiKey
BVNLWJVULAZODX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80 °C
-
沸点:269.28°C (estimate)
-
密度:1.37±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:82.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:212.二硫醇。第二十一部分。环状三硫代碳酸酯中的二硫醇摘要:DOI:10.1039/jr9600001030
-
作为产物:描述:参考文献:名称:Oae, Shigeru; Inubushi, Yoichi; Yoshihara, Masakuni, Phosphorus, Sulfur and Silicon and the Related Elements, 1995, vol. 103, # 1-4, p. 101 - 110摘要:DOI:
-
作为试剂:描述:(Z)-4-methyl-8-oxo-non-4-enal 在 5,6-二氢-4H-1,3-二噻英-2-硫酮 、 氢氧化钾 、 sodium tetrahydroborate 、 三氟乙酸 、 亚磷酸三乙酯 作用下, 以 二氯甲烷 为溶剂, 反应 6.5h, 生成 (2'S,4'aR,8'aR)-2',8'a-dimethylspiro[1,3-dithiane-2,5'-3,4,4a,6,7,8-hexahydro-2H-chromene]参考文献:名称:Mizyuk, V. L., Journal of Organic Chemistry USSR (English Translation), 1988, vol. 24, # 6, p. 1098 - 1107摘要:DOI:
文献信息
-
Studies on the synthesis of vitamin B-12. 3作者:Robert V. Stevens、Jun H. Chang、Richard Lapalme、Steven Schow、Markus G. Schlageter、Rafael Shapiro、Harold N. WellerDOI:10.1021/ja00364a043日期:1983.12Synthese de quatre precurseurs de la vitamine B12 a partir du camphre dextrogyre et levogyreSynthese de quatre precurseurs de la Vitamine B12 a partir du camphre dextrogyre et levogyre
-
Generation of 1,2-ethanebis(trithiocarbonic acid) dianion from 2,2′-[1,2-ethanediylbis(thio)]- bis-1,3-dithiolane and its reaction with alkyl halides作者:Shigeo Tanimoto、Tatuo Oida、Kouhel Hatanaka、Toyonari SugimotoDOI:10.1016/s0040-4039(01)92515-8日期:1981.1Generation of 1,2-ethanbis(trithiocarbonic acid) dianion from 2,2′-[1,2-ethanediylbis (thio)]bis-1,3-dithiolane and its reaction with alkyl halldes were investigated.
-
Thiophilic addition of organolithiums to trithiocarbonate oxides (sulfines)作者:Catherine Leriverend、Patrick Metzner、Antonella Capperucci、Alessandro Degl'InnocentiDOI:10.1016/s0040-4020(96)01071-x日期:1997.1Reaction of trithiocarbonates with meta-chloroperoxybenzoic acid in CH2Cl2 at 0°C affords the corresponding S-oxides. These sulfines are relatively stable compounds which can be purified by chromatography. They react readily with organolithiums in THF at −78°C in a thiophilic manner to give carbanions which are stabilized by three sulfur groups. Hydrolysis affords trithioorthoester oxides. The thermal
-
An efficient one-pot approach to the synthesis of symmetric trithiocarbonates from carbon disulfide and alkyl halides using imidazole作者:Mohammad Soleiman-Beigi、Zahra TaheriniaDOI:10.1080/17415993.2014.919296日期:2014.9.3A novel method is reported for the synthesis of symmetric dialkyl and cyclic (5, 6 and 7 member) trithiocarbonates from alkyl halides and carbon disulfide in the presence of imidazole and water in DMSO under mild reaction conditions. Imidazole is used as an inexpensive, non-toxic and readily available catalyst in this procedure. GRAPHICAL ABSTRACT
-
Convenient Synthesis of Alkanediyl Bis(alkyl trithiocarbonate)s from Alkanedithiols with Alkyl Halides and Carbon Disulfide in the Presence of Phase-Transfer Catalyst作者:Akira Sugawara、Ken Hasegawa、Ken-ichi Suzuki、Yukio Takahashi、Ryu SatoDOI:10.1246/bcsj.60.435日期:1987.1Alkanediyl bis(alkyl trithiocarbonate)s were conveniently synthesized by treating alkanedithiols with alkyl halides and carbon disulfide in the presence of a phase-transfer catalyst.
表征谱图
-
氢谱1HNMR
-
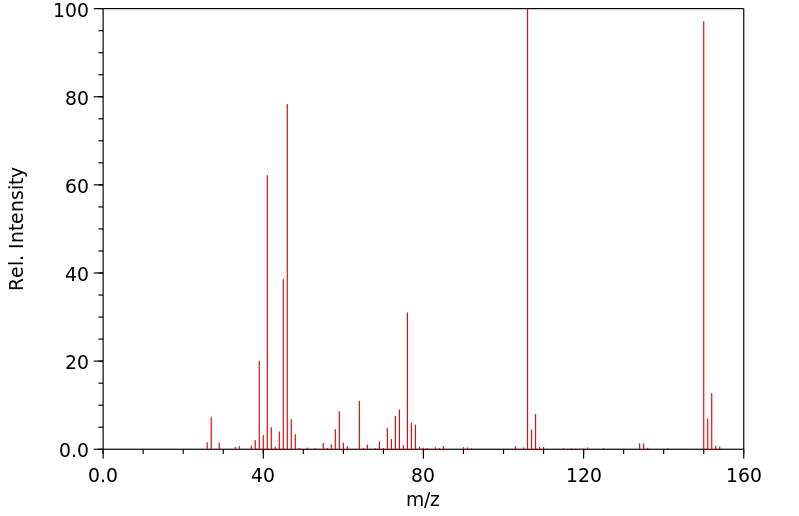
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
硫化膦,1,3-二硫烷-2-基甲基苯基-
硅烷,三甲基(2-甲基-1,3-二硫烷-2-基)-
沙丙喋呤中间体
四氢-1,2-二噻英
反式-1,2-二噻烷-4,5-二醇1,1-二氧化物
八氟-1,4-二噻烷
二(1,3-二噻烷-2-基)甲烷-D
二(1,3-二噻烷-2-基)甲烷
丁二腈,2,3-二[(1,1-二甲基乙基)硫代]-2,3-二(1,3-二硫烷-2-基甲基)-
N-乙基-1,3-二噻烷-2-亚胺
N-(1,3-二硫杂环戊-2-亚基)氨基磷酸二甲酯
N,N’-1,6-己烷二基双氨基甲酸双(1,3-二噻烷-2-基甲基)酯
5alpha-[N-(亚硝基氨基甲酰)-N-(2-氯乙基)氨基]-2beta-甲基-1,3-二噻烷1,1,3,3-四氧化物
5,6-二氢-4H-1,3-二噻英-2-硫酮
4-甲基-2,6,7-三硫杂二环[2.2.2]辛烷
4-(丙氧基甲基)-2,6,7-三硫杂二环[2.2.2]辛烷
3-(1,3-二噻烷-5-基)-1-(2-氟乙基)-1-亚硝基脲
3-(1,3-二噻烷-2-亚基)-2,4-戊二酮
3,3-二甲基二环[2.2.1]庚烷-2-甲醇
2-苯基-1,3-二噻烷锂盐
2-苯基-1,3-二噻烷
2-脱氧-D-阿拉伯糖-己糖亚丙基二硫代缩醛
2-甲基-1,3-二噻烷
2-戊基-1,3-二噻烷
2-异丙基-1,3-二噻烷
2-异丁基-1,3-二噻烷
2-乙炔基-1,3-二噻烷
2-乙基-1,3-二噻烷
2-三甲基硅基-1,3-二噻吩
2-(叔丁基二甲基甲硅烷基)-1,3-二噻烷
2-(三异丙基甲硅烷基)-1,3-二噻烷
2-(3,4-二羟基苯基)-5,7-二羟基-6-[(2S,3R,4R,5S,6R)-3,4,5-三羟基-6-(羟甲基)四氢-2H-吡喃-2-基]-8-[(2S,3R,4S,5S)-3,4,5-三羟基四氢-2H-吡喃-2-基]-4H-色烯-4-酮(non-preferredname)
2-(1,3-二噻烷-2-基)乙醇
2,5-二甲基-2,5-二羟基-1,4-二噻烷
2,5-二甲基-2,5-二羟基-1,4-二噻烷
2,5-二乙氧基-1,4-二噻烷
2,2’-乙烯双(1,3-二噻烷)
2,2-双(三甲基硅基)二噻烷
2,2-二氟-1,3-二噻烷
2,2'-(1,2-亚苯基)二(1,3-二噻烷)
1-(2-氯乙基)-3-(2alpha-甲基-1,3-二噻烷-5alpha-基)-3-亚硝基脲
1-(2-氯乙基)-3-(1,3-二噻烷-5-基)-1-亚硝基脲
1-(2-氯乙基)-1-亚硝基-3-(1,1,3,3-四氧代-1,3-二噻烷-5-基)脲
1-(2-氟乙基)-1-亚硝基-3-(1,1,3,3-四氧代-1,3-二噻烷-5-基)脲
1-(1,3-二噻烷-2-基)乙酮
1-(1,3-二噻烷-2-基)-2-环己烯-1-醇
1-(1,3-二噻烷-2-基)-2,2,2-三氟乙烷酮
1,8-二羟基-2,9-二硫杂三环[8.4.0.03,8]十四烷
1,5,7,11-四硫杂螺[5.5]十一烷
1,4-苯并二噻英,八氢-