二硒化,二-1-萘基 | 1787-80-0
中文名称
二硒化,二-1-萘基
中文别名
——
英文名称
2-bis[3,5bis(trifluoromethyl)phenyl]diselane
英文别名
1,2-di(naphthalen-1-yl)diselane;di(1-naphthyl) diselenide;1,1'-dinaphthyl diselenide;bis(1-naphthyl) diselenide;dinaphthyl diselenide;1,2-di(naphthalen-2-yl)diselane;Diselenide, di-1-naphthalenyl;1-(naphthalen-1-yldiselanyl)naphthalene
CAS
1787-80-0
化学式
C20H14Se2
mdl
——
分子量
412.251
InChiKey
XFUCRHYSJRXATP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:87-88 °C
-
沸点:552.9±33.0 °C(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.27
-
重原子数:22
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Laser-flash photolysis of naphthyl diselenides; reactivities of naphthylseleno radicals摘要:Transient absorption spectra of 1-naphthylseleno (1-NaphSe(.)), and 2-naphthylseleno (2-NaphSe(.)) radicals, which are generated by laser-flash photolysis of the corresponding diselenides, were observed. The reactions of 1-NaphSe(.), and 2-NaphSe(.) with 2-methyl-1,3-butadiene and alpha-methylstyrene were investigated by following the decay rates of these seleno radicals. By both steady-state and laser-flash photolysis, it is proved that these seleno radicals add to alkenes in a reversible manner. The reaction rate constants for such reversible addition reactions were determined by conducting the reaction in the presence of O(2), which traps selectively the carbon-centered radicals formed by the addition reaction of the seleno radicals to the alkenes. The reactivity of 2-NaphSe(.) is higher than that of 1-NaphSe(.), both of which are less reactive than PhSe .. These reactivities were interpreted with the properties of SOMO calculated by MO method. (C) 1998 John Wiley & Sons, Inc.DOI:10.1002/(sici)1097-4601(1998)30:3<193::aid-kin4>3.0.co;2-n
-
作为产物:参考文献:名称:The direct synthesis of symmetrical disulfides and diselenides by metal–organic framework MOF-199 as an efficient heterogenous catalyst摘要:MOF-199被用作高效的杂化催化剂,用于在聚乙二醇(PEG)中从芳基卤化物和元素S/Se一锅法合成有机二硫化物/二硒化物,产率良好至优异。DOI:10.1039/c5ra16879a
文献信息
-
Nucleophilic cleavage of lactones and esters with zinc selenolates prepared from diselenides in the presence of Zn/AlCl3作者:Mohammad Nazari、Barahman MovassaghDOI:10.1016/j.tetlet.2008.11.036日期:2009.1The utility of zinc selenolates for effecting nucleophilic cleavage of simple lactones and esters has been investigated. When zinc selenolate generated via Zn/AlCl3-promoted cleavage of diselenides was reacted with simple lactones and esters, efficient nucleophilic alkyl–oxygen bond cleavage proceeded generating the corresponding carboxylic acids in moderate to excellent yields.
-
Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature
-
Regioselective, Solvent- and Metal-Free Chalcogenation of Imidazo[1,2-<i>a</i> ]pyridines by Employing I<sub>2</sub> /DMSO as the Catalytic Oxidation System作者:Jamal Rafique、Sumbal Saba、Alisson R. Rosário、Antonio L. BragaDOI:10.1002/chem.201600800日期:2016.8.8molecular‐iodine‐catalyzed chalcogenations (S and Se) of imidazo[1,2‐a]pyridines were achieved by using diorganoyl dichalcogenides under solvent‐free conditions. This approach afforded the desired products that had been chalcogenated regioselectively at the C3 position in up to 96 % yield by using DMSO as an oxidant, in the absence of a metal catalyst, and under an inert atmosphere. This mild, green approach allowed
-
Visible-light-induced metal and reagent-free oxidative coupling of <i>sp</i><sup>2</sup> C–H bonds with organo-dichalcogenides: synthesis of 3-organochalcogenyl indoles作者:Vandana Rathore、Sangit KumarDOI:10.1039/c9gc00007k日期:——and halo either on indoles or aryl dichalcogenides showed amenability to the developed reaction. Furthermore, thiocyanation of the sp2 C–H bonds of indoles has been accomplished by this visible light induced method. A mechanistic understanding by UV-visible, EPR spectroscopy, and cyclic voltammetry suggests that light induces electron transfer from the electron rich arene to oxygen providing an arene在这里,已经提出了一种独特的可见光诱导方法,用于将吲哚和苯胺的sp 2 C–H键进行有机硫属化,使用二芳基二卤化碳(S,Se和Te)和氧气作为氧化剂,避免了光催化剂,碱,室温下,在丙酮中加入催化剂和试剂。这种良性方案允许以良好或优异的产率获得各种3-芳基硒基吲哚,3-芳基硫代吲哚,甚至3-芳基碲代吲哚。吲哚或芳基二卤化物上的各种官能团,即甲氧基和卤素,都显示出对已开发的反应的适应性。此外,sp 2的硫氰化吲哚的CH键已通过这种可见光诱导方法完成。通过紫外可见光,EPR光谱和循环伏安法进行的机理理解表明,光诱导电子从富电子的芳烃向氧的转移,从而提供芳烃自由基阳离子和超氧自由基阴离子。随后,自由基阳离子与芳基二硫代氰化物的反应提供了二芳基硫属取代基阳离子,其在去除质子后得到不对称的3-吲哚基芳基硫属化物。
-
Direct, Metal-free C(sp<sup>2</sup> )−H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO<sub>3</sub>作者:Jamal Rafique、Sumbal Saba、Marcelo S. Franco、Luana Bettanin、Alex R. Schneider、Lais T. Silva、Antonio L. BragaDOI:10.1002/chem.201705404日期:2018.3.15synthesis of 3‐Se/S‐indoles and imidazo[1,2‐a]pyridines through direct C(sp2)−H bond chalcogenation of heteroarenes with half molar equivalents of different dichalcogenides, using KIO3 as a non‐toxic, easy‐to‐handle catalyst and a stoichiometric amount of glycerol. The reaction features are high yields, based on atom economy, easy performance on gram‐scale, metal‐ and solvent‐free conditions as well as applicability
表征谱图
-
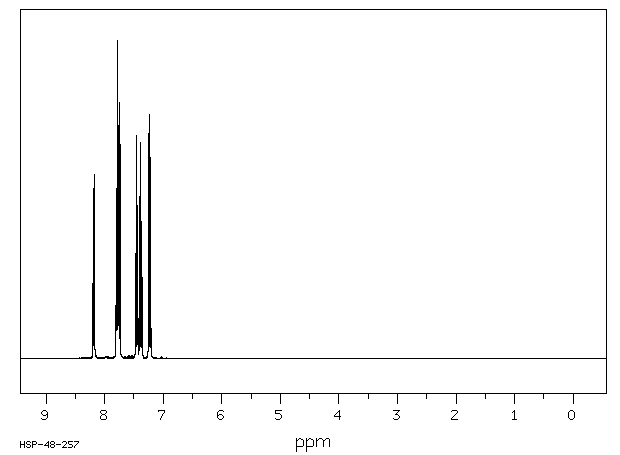
氢谱1HNMR
-
质谱MS
-
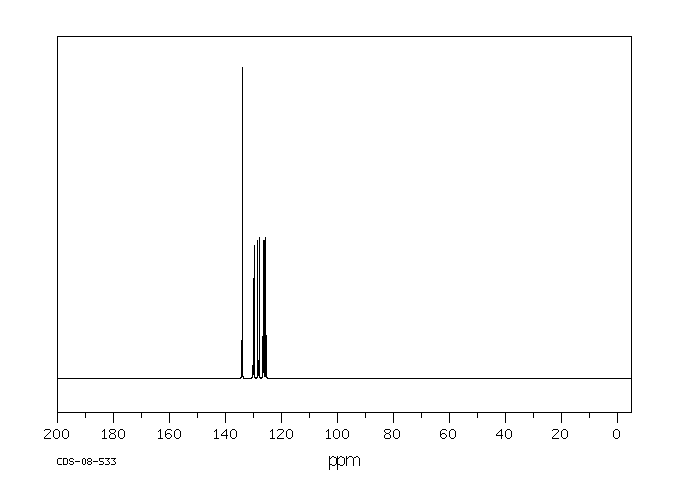
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮