3-溴庚烷 | 1974-05-6
中文名称
3-溴庚烷
中文别名
3-庚基溴
英文名称
3-bromoheptane
英文别名
(+/-)-3-Brom-heptan;3-heptyl bromide
CAS
1974-05-6
化学式
C7H15Br
mdl
——
分子量
179.1
InChiKey
MLHXKYLLJRLHGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:61-62°C 18mm
-
密度:1,14 g/cm3
-
闪点:61-62°C/18mm
-
保留指数:1022;1036;1048;988
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S23,S24/25
-
危险类别码:R10
-
包装等级:III
-
危险类别:3
-
危险品运输编号:1993
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
3-溴庚烷
模块 1. 化学品
产品名称: 3-Bromoheptane
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-溴庚烷
百分比: >90.0%(GC)
CAS编码: 1974-05-6
俗名: 3-Heptyl Bromide
分子式: C7H15Br
3-溴庚烷
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
3-溴庚烷
模块 9. 理化特性
pH: 无数据资料
熔点: 无资料
沸点/沸程 140 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.14
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
3-溴庚烷
模块 14. 运输信息
正式运输名称: 易燃液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3-Bromoheptane
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-溴庚烷
百分比: >90.0%(GC)
CAS编码: 1974-05-6
俗名: 3-Heptyl Bromide
分子式: C7H15Br
3-溴庚烷
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
3-溴庚烷
模块 9. 理化特性
pH: 无数据资料
熔点: 无资料
沸点/沸程 140 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.14
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
3-溴庚烷
模块 14. 运输信息
正式运输名称: 易燃液体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:New Mesogenic Compounds Exhibiting a Thermotropic Optically Isotropic Phase摘要:The only two series of carboxylic acids, 3-nitro- or 3-cyano-4-alkoxybiphenyl-4-carboxylic acid, are known to exhibit the optically isotropic phase (SID). Now we synthesised 4-(branched alkoxy)-anilinebenzylidene-4'-carboxylic acid series, all being racemic, and investigated their mesophase properties by means of DSC, optical microscopy and X-ray measurement. A, a result, some branched azomethine derivatives were found to form a SD phase. We also synthesised cinnamic acid derivatives with branched alkoxy tail that were turned out to exhibit either SD phase or hexagonal columnar phase. We tried biphenyl or azobenzene core analogues which were found to produce a highly ordered smectic phase or no mesophase, respectively. At this stage, we suppose it plays an important role that the branch is introduced into the terminal chain and a certain dipole moment is located within the mesogenic core. These factors may allow the peculiar molecular packing that causes an optically isotropic property.DOI:10.1080/10587250108025056
-
作为产物:参考文献:名称:羧酸银盐与卤素的反应。摘要:DOI:10.1021/ja01192a502
文献信息
-
A general N-alkylation platform via copper metallaphotoredox and silyl radical activation of alkyl halides作者:Nathan W. Dow、Albert Cabré、David W.C. MacMillanDOI:10.1016/j.chempr.2021.05.005日期:2021.7The catalytic union of amides, sulfonamides, anilines, imines, or N-heterocycles with a broad spectrum of electronically and sterically diverse alkyl bromides has been achieved via a visible-light-induced metallaphotoredox platform. The use of a halogen abstraction-radical capture (HARC) mechanism allows for room temperature coupling of C(sp3)-bromides using simple Cu(II) salts, effectively bypassing通过可见光诱导的金属光氧化还原平台实现了酰胺、磺胺、苯胺、亚胺或N-杂环与广谱电子和空间多样化烷基溴的催化结合。卤素提取-自由基捕获 (HARC) 机制的使用允许使用简单的 Cu(II) 盐在室温下耦合 C( sp 3 )-溴化物,有效绕过通常与热诱导 S N 2 或 S相关的过高障碍N 1 N-烷基化。这种区域和化学选择性方案与 >10 类药物相关的N兼容- 亲核试剂,包括已建立的药剂,以及结构多样的伯、仲和叔烷基溴。此外,通过将N-亲核试剂与环丙基溴和未活化的烷基氯(与亲核取代途径不相容的底物)结合,突出了 HARC 方法与传统惰性偶联伙伴结合的能力。初步的机械实验验证了该平台的双重催化、开壳性质,这使得在传统的基于卤化物的N-烷基化系统中无法实现的反应性成为可能。
-
FLUORENE-TYPE COMPOUND, PHOTOPOLYMERIZATION INITIATOR COMPRISING SAID FLUORENE-TYPE COMPOUND, AND PHOTOSENSITIVE COMPOSITION CONTAINING SAID PHOTOPOLYMERIZATION INITIATOR申请人:Daito Chemix Corporation公开号:US20150259321A1公开(公告)日:2015-09-17According to an embodiment of the present invention, there is provided a novel fluorene-based compound and a photopolymerization initiator using the fluorene-based compound. The fluorene-based compound according to an embodiment of the present invention is represented by the general formula (1). A photopolymerization initiator having additionally high sensitivity can be provided by using the fluorene-based compound. In addition, a photopolymerization initiator that can impart additionally excellent characteristics can be provided by appropriately selecting, for example, a substituent.
-
Biocatalytic Construction of Quaternary Centers by Aldol Addition of 3,3-Disubstituted 2-Oxoacid Derivatives to Aldehydes作者:Roser Marín-Valls、Karel Hernández、Michael Bolte、Teodor Parella、Jesús Joglar、Jordi Bujons、Pere ClapésDOI:10.1021/jacs.0c09994日期:2020.11.183-methyl-2-oxobutanoate hydroxymethyltransferase from E. coli (KPHMT) and variants thereof. The 3,3,3-trisubstituted 2-oxoacids thus produced were converted into 2-oxolactones and 3-hydroxy acids and directly to ulosonic acid derivatives, all bearing gem-dialkyl, gem-cycloalkyl, and spirocyclic quaternary centers. In addition, some of these reactions use a single enantiomer from racemic nucleophiles to afford
-
Direct Bromination and Iodination of Non-Activated Alkanes by Hypohalite Reagents作者:Thomas Wirth、Raúl MontoroDOI:10.1055/s-2005-865322日期:——The direct functionalisation of alkanes through bromination and iodination has been successfully achieved. The combination of stoichiometric mixtures of elemental halogen and sodium alkoxides leads to the formation of alkyl hypobromites and hypoiodites as reagents. The halogenation occurs without external photostimulation under thermal reaction conditions.
-
Reductive coupling of benzyl oxalates with highly functionalized alkyl bromides by nickel catalysis作者:Xiao-Biao Yan、Chun-Ling Li、Wen-Jie Jin、Peng Guo、Xing-Zhong ShuDOI:10.1039/c8sc00609a日期:——Coupling reactions involving non-sulfonated C–O electrophiles provide a promising method for forming C–C bonds, but the incorporation of functionalized or secondary alkyl groups remains a challenge due to the requirement for well-defined alkylmetal species. In this study, we report a reductive nickel-catalyzed cross-coupling of benzyl oxalates with alkyl bromides, using oxalate as a new leaving group
表征谱图
-
氢谱1HNMR
-
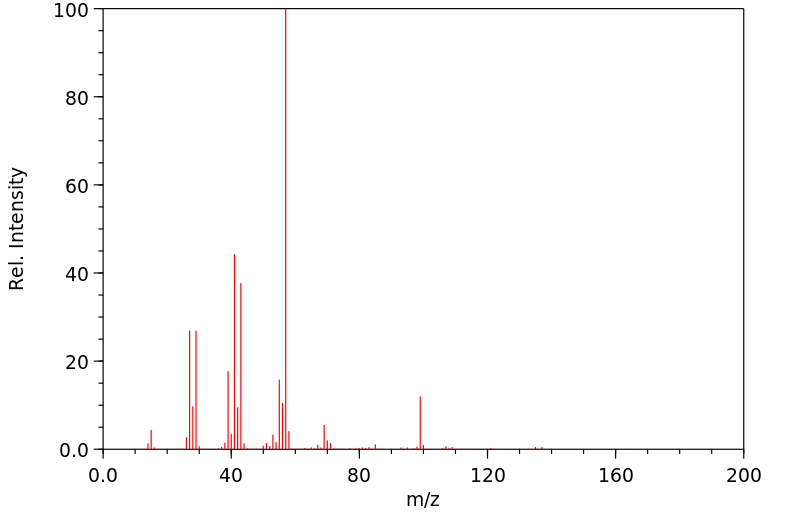
质谱MS
-
碳谱13CNMR
-
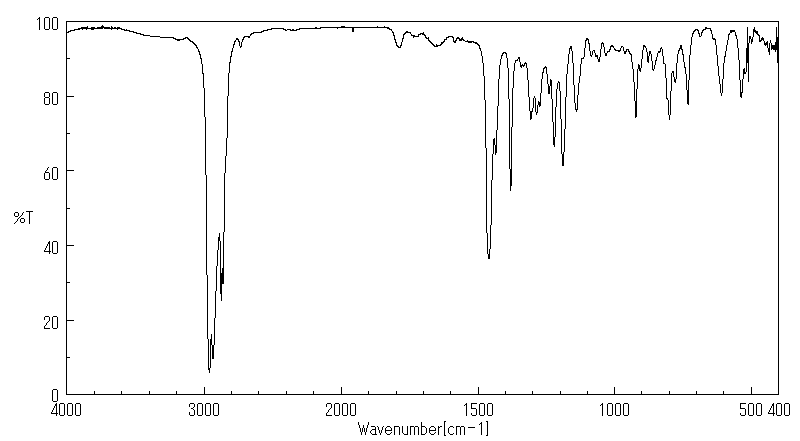
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4