3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪 | 1046-56-6
中文名称
3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪
中文别名
5,6-联苯基-3-(2-吡啶基)-1,2,4-三吖嗪
英文名称
5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine
英文别名
3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine;5,6-diphenyl-3-pyridin-2-yl-1,2,4-triazine
CAS
1046-56-6
化学式
C20H14N4
mdl
MFCD00006462
分子量
310.358
InChiKey
OTMYLOBWDNFTLO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:191-193 °C (lit.)
-
沸点:440.52°C (rough estimate)
-
密度:1.2028 (rough estimate)
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:24
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
RTECS号:XY8598150
-
海关编码:2933990090
-
安全说明:S26,S36
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| Name: | 3-(2-Pyridyl)-5 6-Diphenyl-1 2 4-Triazine 99+% Material Safety Data Sheet |
| Synonym: | 5,6-Diphenyl-3-(2-Pyridyl)-1,2,4-Triazine; PDT |
| CAS: | 1046-56-6 |
Synonym:5,6-Diphenyl-3-(2-Pyridyl)-1,2,4-Triazine; PDT
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1046-56-6 | 3-(2-Pyridyl)-5,6-Diphenyl-1,2,4-Triaz | 99+% | 213-878-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1046-56-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 191.00 - 193.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: slightly soluble
Specific Gravity/Density:
Molecular Formula: C20H14N4
Molecular Weight: 310.35
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong acids.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1046-56-6: XY8598150 LD50/LC50:
Not available.
Carcinogenicity:
3-(2-Pyridyl)-5,6-Diphenyl-1,2,4-Triazine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1046-56-6: No information available.
Canada
CAS# 1046-56-6 is listed on Canada's DSL List.
CAS# 1046-56-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1046-56-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 6-phenyl-3-(2'-pyridyl)-1,2,4-triazine-4-oxide 453556-95-1 C14H10N4O 250.26
反应信息
-
作为反应物:描述:3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪 以26%的产率得到参考文献:名称:FIGEYS, H. P.;MATHY, A., TETRAHEDRON LETT., 1981, 22, N 15, 1393-1396摘要:DOI:
-
作为产物:描述:2-cyano-pyridine hydrochloride 在 一水合肼 、 sodium nitrite 作用下, 以 甲苯 为溶剂, 反应 72.0h, 生成 3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪参考文献:名称:Diels-alder reactions with inverse electron demand. II. The reaction of benzamidine with π-deficient heteroaromatic compounds.摘要:DOI:10.1016/s0040-4039(01)90330-2
-
作为试剂:描述:参考文献:名称:Versatile solid-phase thiolytic reduction of azido and N-Dts groups in the synthesis of haemoglobin (67–76) O-glycopeptides and photoaffinity labelled analogues to study glycan T-cell specificity摘要:利用糖基化结构单元 1â4 和 7â21 通过多柱肽合成法合成了一系列 O-糖基化肽和光亲和标记的小鼠血红蛋白衍生十肽 Hb(67â76)的糖肽类似物 VITAFNEGLK,它能很好地与 MHC II 类 Ek 分子结合,并且对 CBA/J 小鼠无免疫原性。通过在十肽的中心位置 Asn-72 引入不同的肿瘤相关碳水化合物分子 [δ-D-GlcNAc-O-Ser/Thr、δ-D-GalNAc-O-Ser/Thr(TN 抗原)核心 1(T 抗原)、核心 2、核心 3 和核心 4],将非免疫原性肽 VITAFNEGLK 转化为免疫原。 以往的研究表明,T 细胞可能能够识别部分由聚糖定义的表位,并可能与 T 细胞受体直接接触。为了研究聚糖与 T 细胞受体相互作用的特异性,我们合成了一系列在碳水化合物氨基功能上用 2- 叠氮苯甲酰胺标记的相应聚糖肽。聚糖结构随 O-GlcNAc、T 和 TN 抗原分子以及异构体构型的不同而变化。自始至终,含 N-二硫代丁二酰基和叠氮官能团的结构单元 1、2、7、8、11、12、13、16、18 和 20 都可以通过以下两种方法实现高效还原:(i) 在溶液中用 Zn 在 THFâHOAcâAc2O 中同时原位还原;或 (ii) 在固相上用二异丙基乙胺和过量的二硫苏糖醇或δ-巯基-N-甲基乙酰胺处理。对树脂结合的糖肽进行 N-乙酰化,可得到 O-糖肽 24、25 和 31â36。在使用 N-乙酰化结构单元 3、4、9、10、14、15、17、19 和 21 时,不需要在固相上进一步修饰碳水化合物分子。此外,在合成 O-连接糖肽 24、25 和 31â36 时,还进行了固相还原比较研究,采用的是 1â4 和 7â21 中的任何一种构建模块。光亲和标记的糖肽 39â45 是通过使用构建模块 1、2、7、8 和 11â13 合成的,方法是用二硫苏糖醇进行硫醇分解还原叠氮或 N-Dts 官能,然后将活化的光亲和标记 38 与树脂结合的糖肽的甘氨酸基偶联。通过一维和二维 1H NMR 光谱以及电喷雾质谱法,对合成的粘蛋白 O 型糖肽 24、25 和 31â36 以及光亲和标记的类似物 39â45 进行了全面鉴定。DOI:10.1039/a606725e
文献信息
-
Studies on the interactions of 5-<i>R</i>-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels–Alder reaction <i>versus</i> aryne-mediated domino process作者:Dmitry S. Kopchuk、Igor L. Nikonov、Albert F. Khasanov、Kousik Giri、Sougata Santra、Igor S. Kovalev、Emiliya V. Nosova、Sravya Gundala、Padmavathi Venkatapuram、Grigory V. Zyryanov、Adinath Majee、Oleg N. ChupakhinDOI:10.1039/c8ob00847g日期:——aza-Diels–Alder products or 1,2,4-triazine ring rearrangement (domino) products as major ones depending on the nature of both the substituents at the C5 position of the 1,2,4-triazine core or in the aryne moiety. The structures of the key products were confirmed based on X-ray data. Based on the density functional theoretical (DFT) studies of the Diels–Alder transition state geometries, the influence of the nature
-
Kinetics and mechanism of base hydrolysis of tris(3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine)iron(II) in aqueous and micellar media作者:Rajesh Bellam、G. Ganga Raju、Nageswara Rao Anipindi、Deo JaganyiDOI:10.1007/s11243-015-0018-z日期:2016.4decrease with increasing ionic strength in aqueous and CTAB media, whereas SDS medium shows little ionic strength effect. The rate also increases with CTAB concentration but decreases with SDS. The specific rate constant, k and thermodynamic parameters (Ea, ΔH#, ΔS# and ΔG#) have also been evaluated. The near equal values of ΔG# obtained in aqueous and CTAB media suggest that these reactions occur essentially三(3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪)铁(II)碱水解动力学,$$ \textFe(PDT)}}_ 3 }^2 + } $$Fe(PDT)32+ 已在 25、35 和 45 °C 下在伪一级条件下在十六烷基三甲基溴化铵 (CTAB) 和十二烷基硫酸钠 (SDS) 介质中进行了研究,即 $$ [ \textOH}}^ - } ]\gg [\textFe(PDT)}}_ 3}^2 + } ] $$[OH-]≫[Fe(PDT) )32+]。反应在底物 $$ \textFe(PDT)}}_ 3}^2 + } $$Fe(PDT)32+ 和氢氧根离子中都是一级反应。在水介质和 CTAB 介质中,速率随着离子强度的增加而降低,而 SDS 介质显示出的离子强度影响很小。该速率也随 CTAB 浓度而增加,但随 SDS 而降低。还评估了比速率常数、k 和热力学参数(Ea、ΔH#、ΔS#
-
Broadening the scope of 1,2,4-triazine synthesis by the application of microwave technology作者:Zhijian Zhao、William H. Leister、Kimberly A. Strauss、David D. Wisnoski、Craig W. LindsleyDOI:10.1016/s0040-4039(02)02845-9日期:2003.2By the application of microwave technology, a general protocol has been developed for the rapid synthesis of diverse 3,5,6-trisubstituted 1,2,4-triazines in excellent yield and purity, including many previously unknown 3-heterocyclic-1,2,4-triazines.通过微波技术的应用,已开发出通用协议,可以以优异的收率和纯度快速合成各种3,5,6-三取代的1,2,4-三嗪,包括许多以前未知的3-杂环-1,2 ,4-三嗪。
-
Efficient Preparation of Pyridinyl-1,2,4-Triazines via Telescoped Condensation with Diversely Functionalized 1,2-Dicarbonyls作者:Serene Tai、Sydney V. Marchi、Jesse D. CarrickDOI:10.1002/jhet.2374日期:2016.7useful 3‐pyridin‐2‐yl‐1,2,4‐triazines (2 novel) is demonstrated via telescoped condensation with the requisite 1,2‐dicarbonyl in two‐pots without additives, differentially extractive work‐up procedures, or recrystallization. Additional efforts in this area have demonstrated the utility of polar aprotic solvents for the preparation of nine functionalized pyridinyl‐2,6‐bis‐1,2,4‐triazines (4 novel) directly有效化学选择性提取次minor系元素的材料的开发仍然是分离科学领域研究工作的前沿。由于与超铀元素element有良好的络合作用,该领域经常使用源自氮供体支架的路易斯碱性络合物。在目前的工作中,通过在两个罐中与所需的1,2-二羰基进行缩合缩合缩合,证明了制备八种有用的3-吡啶-2--2-基1,2,4-三嗪(2种新颖)的有效方法。添加剂,差异萃取后处理程序或重结晶。在这一领域的进一步努力证明了极性非质子溶剂在制备九种官能化吡啶基-2-6- bis中的实用性直接从必需的2,6-吡啶二甲腈中得到1,2,4-三嗪(4种新颖化合物),整个四个步骤的总收率为49-99%。本文报道了这些重要材料的简化制备方法和详细的合成步骤。
-
Synthesis, crystal structure, bovine serum albumin binding studies of 1,2,4-triazine based copper(I) complexes作者:Larica Pathaw、Themmila Khamrang、Arunkumar Kathiravan、Marappan VelusamyDOI:10.1016/j.molstruc.2020.127821日期:2020.5Abstract A series of new copper complexes have been synthesized and completely characterized by pivotal analytical techniques. The coordination geometry around copper(I) complex was best described as distorted tetrahedral geometry. The binding of bovine serum albumin with Cu(I) complexes are also been investigated. The Stern–Volmer analysis on quenching data exhibits the presence of the static quenching
表征谱图
-
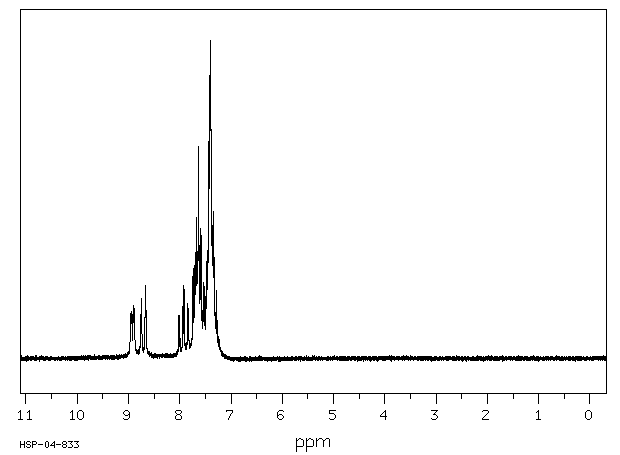
氢谱1HNMR
-
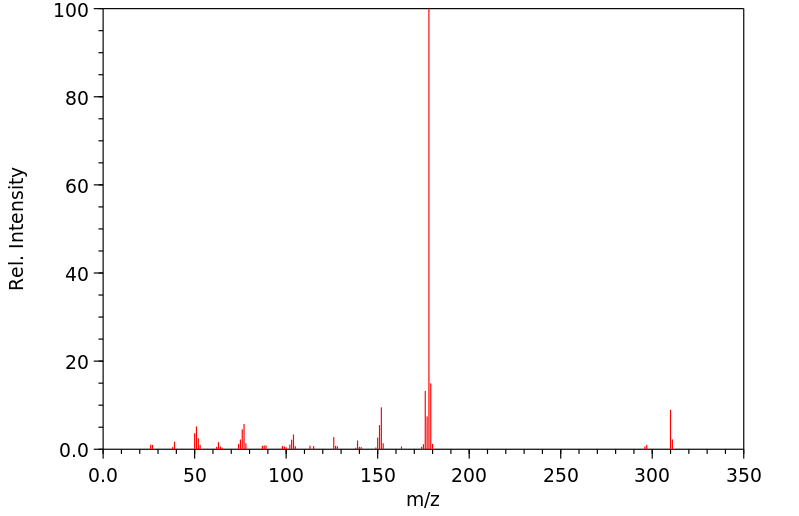
质谱MS
-
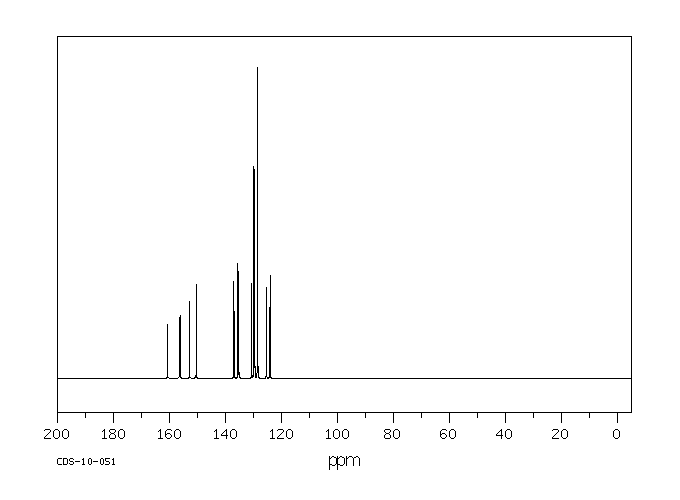
碳谱13CNMR
-
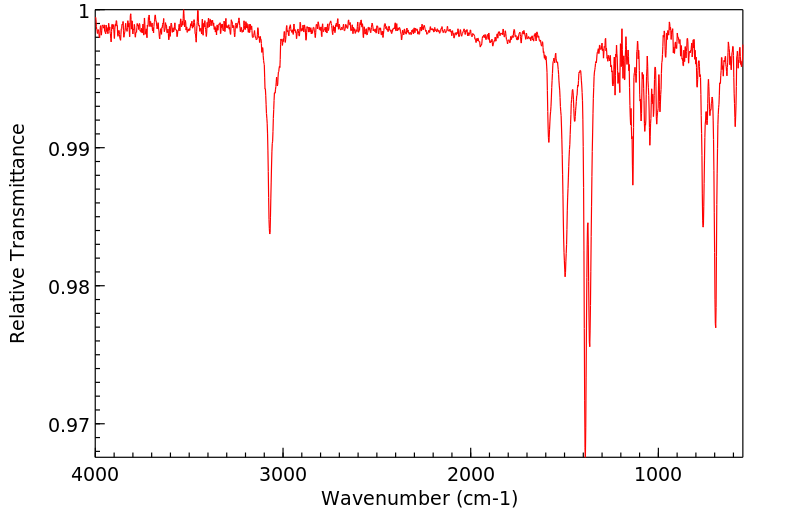
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4