丁基黄原酸钾 | 871-58-9
中文名称
丁基黄原酸钾
中文别名
丁基二硫代碳酸钾;二硫代碳酸-O-丁酯钾盐
英文名称
potassium butylxanthate
英文别名
potassium n-butyl xanthate;potassium O-butylxanthate;potassium O-butyldithiocarbonate;potassium n-butyl xanthogenate;potassium O-butyl carbonodithioate;potassium O-n-butyl dithiocarbonate;potassium;butoxymethanedithioate
CAS
871-58-9
化学式
C5H9OS2*K
mdl
——
分子量
188.356
InChiKey
OMKVZYFAGQKILB-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70 °C (decomp)
-
溶解度:少许溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):-1.36
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:42.3
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:4.2
-
安全说明:S20/21,S36/37/39
-
危险类别码:R20/21/22,R36/38
-
海关编码:2930902000
-
危险品运输编号:3342
-
RTECS号:FG1223000
-
包装等级:II
-
危险类别:4.2
-
储存条件:密封冷藏
SDS
Potassium Butylxanthate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Potassium Butylxanthate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Acute toxicity (Inhalation) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful by inhalation and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
Avoid breathing.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Potassium Butylxanthate
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Potassium Butylxanthate
Percent: >95.0%(T)
CAS Number: 871-58-9
Synonyms: Butylxanthic Acid Potassium Salt
Chemical Formula: C5H9KOS2
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Potassium Butylxanthate
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Pale yellow green
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Heat-sensitive, Light-sensitive
Conditions to avoid:
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
ihl-rat LC50:7690 mg/m3/2H
Acute Toxicity:
orl-rat LD50:456 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: FG1223000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Potassium Butylxanthate
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Potassium Butylxanthate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 4
Acute toxicity (Oral)
Acute toxicity (Inhalation) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Harmful by inhalation and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
Avoid breathing.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Potassium Butylxanthate
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Potassium Butylxanthate
Percent: >95.0%(T)
CAS Number: 871-58-9
Synonyms: Butylxanthic Acid Potassium Salt
Chemical Formula: C5H9KOS2
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Potassium Butylxanthate
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Pale yellow green
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Heat-sensitive, Light-sensitive
Conditions to avoid:
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
ihl-rat LC50:7690 mg/m3/2H
Acute Toxicity:
orl-rat LD50:456 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: FG1223000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Potassium Butylxanthate
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:丁基黄原酸钾 在 sodium monochloroacetic acid 、 ammonium hydroxide 作用下, 以 水 为溶剂, 以89%的产率得到O-1-butyl thiocarbamate参考文献:名称:Hairpin Folding Behavior of Mixed α/β-Peptides in Aqueous Solution摘要:The invention of new strategies for the design of protein-mimetic oligomers that manifest the folding encoded in natural amino acid sequences is a significant challenge. In contrast to the a-helix, mimicry of protein beta-sheets is less understood. We report here the aqueous folding behavior of a prototype alpha-peptide hairpin model sequence varied at cross-strand positions by incorporation of 16 different beta-amino acid monomers. Our results provide a folding propensity scale for beta-residues in a protein beta-sheet context as well as high-resolution structures of several mixed-backbone alpha/beta-peptide hairpins in water.DOI:10.1021/ja2002346
-
作为产物:参考文献:名称:Novel thiadiazoles摘要:公式为##STR1##的新型噻二唑,其中R是1至3个碳原子的烷基,W选择自氧和硫,X选择自--O--,--S--和##STR2##,R"选择自氢和1至3个碳原子的烷基,n为1、2、3或4,R'选择自氢,--CN,1至3个碳原子的烷氧基,2至4个碳原子的烷氧羰基,2至4个碳原子的烯基,苯基,其可选地用卤素、1至3个碳原子的烷基和1至3个碳原子的烷氧基中的一个成员或用卤素、1至3个碳原子的烷基和1至3个碳原子的烷氧基中的两个成员取代,具有杀虫和杀线虫作用。公开号:US04069319A1
-
作为试剂:描述:参考文献:名称:Ring-chain tautomeric transformations of the product of diazotization of 5-(2?-aminophenyl)tetrazole摘要:DOI:10.1007/bf00506469
文献信息
-
Copper-catalyzed synthesis of thiazol-2-yl ethers from oxime acetates and xanthates under redox-neutral conditions作者:Zhongzhi Zhu、Xiaodong Tang、Jinghe Cen、Jianxiao Li、Wanqing Wu、Huanfeng JiangDOI:10.1039/c8cc00445e日期:——acetates and xanthates for the synthesis of thiazol-2-yl ethers with remarkable regioselectivity has been developed. Various oxime acetates, whether derived from aryl ketones or alkyl ketones, or natural product cores are suitable for this conversion. Unique dihydrothiazoles were also obtained when both reaction sites were methine. Mechanistic studies indicated that imino copper(III) intermediates were involved
-
Comparative study of the13C nuclear magnetic resonance shifts of carbonyl and thiocarbonyl compounds作者:Alan R. Katritzky、Stanislaw Sobiak、Charles M. MarsonDOI:10.1002/mrc.1260260805日期:1988.8The 13C spectra of nine novel and fifteen known derivatives YC(:X)Z with X = O or S are reported and compared with literature data. Two linear relationships between 13CO and 13CS are established depending on whether or not Y or Z is linked through an O atom to the CX group. General equations for 13CO and 13CS in terms of the attached groups are also deduced. The 13C chemical shifts of n-butyl and n-octyl groups attached to the heteroatoms in this series of compounds are recorded and discussed.
-
Novel Xanthate Complexes for the Size-Controlled Synthesis of Copper Sulfide Nanorods作者:Mundher Al-Shakban、Peter D. Matthews、Geradius Deogratias、Paul D. McNaughter、James Raftery、Inigo Vitorica-Yrezabal、Egid B. Mubofu、Paul O’BrienDOI:10.1021/acs.inorgchem.7b01288日期:2017.8.7We present a simple, easily scalable route to monodisperse copper sulfide nanocrystals by the hot injection of a series of novel copper(I) xanthate single-source precursors [(PPh3)2Cu(S2COR)] (R = isobutyl, 2-methoxyethyl, 2-ethoxyethyl, 1-methoxy-2-propyl, 3-methoxy-1-butyl, and 3-methoxy-3-methyl-1-butyl), whose crystal structures are also reported. We show that the width of the obtained rods is
-
Metal-Free Oxidative Esterification of Ketones and Potassium Xanthates: Selective Synthesis of α-Ketoesters and Esters作者:Xianglin Luo、Runfa He、Qiang Liu、Yanping Gao、Jingqing Li、Xiuwen Chen、Zhongzhi Zhu、Yubing Huang、Yibiao LiDOI:10.1021/acs.joc.9b03272日期:2020.4.17A novel and efficient oxidative esterification for the selective synthesis of α-ketoesters and esters has been developed under metal-free conditions. In the protocol, various α-ketoesters and esters are available in high yields from commercially available ketones and potassium xanthates. Mechanistic studies have proven that potassium xanthate not only promotes oxidative esterification but also provides
-
In Situ Synthesis of PbS Nanocrystals in Polymer Thin Films from Lead(II) Xanthate and Dithiocarbamate Complexes: Evidence for Size and Morphology Control作者:Edward A. Lewis、Paul D. McNaughter、Zhongjie Yin、Yiqiang Chen、Jack R. Brent、Selina A. Saah、James Raftery、Johannes A. M. Awudza、M. Azad Malik、Paul O’Brien、Sarah J. HaighDOI:10.1021/cm504765z日期:2015.3.24film to decompose the precursor. The effect of precursor chemistry has been explored using lead(II) dithiocarbamates, their 1,10-phen adducts, and lead(II) xanthates with different alkyl chain lengths (butyl, hexyl, and octyl). The xanthates were found to be more promising precursors giving control over nanocrystal size and shape on variation of the alkyl chain length. The lead(II) octyl xanthate complex
表征谱图
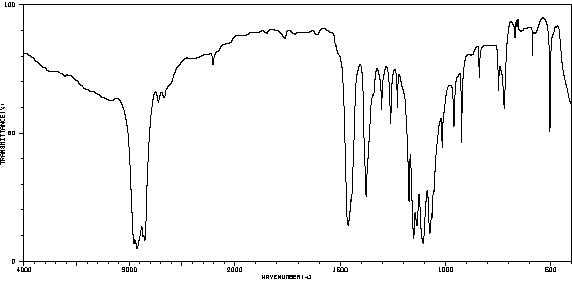
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄原酸环癸酯
高纯三甲基锑
顺式-二氯二(环丙胺)铂(II)
顺式-二氯二(乙二胺)氯化铑(1+)
顺式-二(环己基丁氨合)二氯铂(II)
顺式-二(异丙基氨合)二氯铂(II)
顺式-(2-氨基甲基-1-环戊基氨合)二氯铂(II)
顺二氯二羰基铂(II)
顺-二氯双(乙二胺)氯化铱
雷(酸)汞[含水或水加乙醇≥20]
间碳硼烷-9-硫醇
镍,加合(7:2)钪
镉二(二戊基二硫代氨基甲酸盐)
镁,溴-6-庚烯基-
manganese carbide
butyl manganese bromide
锡烷,氯二环己基-
锡四丁醇
锑,(1:1)混合物和钪
锌叔-丁氧化物
锌,溴-1-丙烯基-,(E)-
锇,加合(2:1)钪
锆酸四丁酯
锂丁酯
锂4-异丙氧基-2-甲基-丁烷-2-醇
锂1-丁醇
锂(三氟甲基)乙炔化物
锂(3-氨基丙基)酰胺
铼五羰基碘化物
铼五羰基
银(I)2-羟基乙烷-1-硫醇盐
铯三氯三羰基锇
铬三乙二胺
铬,五羰基(环己胺)-,(OC-6-22)-
铬,二(乙酰腈)二氯-
铝,加合(3:1)钪
铜-乙二胺络合物
铜(II)乙二胺
铜(I)乙炔化物
铍,环戊-1,3-二烯,溴化
铊N,N-二正丁胺
铊,甲氧基二甲基-
铂(2+)二氯化3-甲基丁烷-1,2-二胺(1:1)
铁(3+)三(1-丁醇)
铁(2+)1,1'-(硫烷二基二-1,1-乙二基)二-2,4-环戊二烯化
铀,三甲基-
钾,[三(三甲基甲硅烷基)甲基]-
钴四异硫氰酸酯
钴,乙烷-1,2-二胺
钠辛基二硫代氨基甲酸酯