氟乙酰胺 | 640-19-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂、强还原剂、强酸和强碱接触。
计算性质
-
辛醇/水分配系数(LogP):-1
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:43.1
-
氢给体数:1
-
氢受体数:2
ADMET
制备方法与用途
氟乙酰胺由氯乙酰胺氟化而得。氟化反应既可以在二甲苯或四氯乙烯等溶剂中进行,也可以采用干法(无溶剂法),即干燥的氯乙酰胺与KF(氟化钾)加热反应。干法操作简便且没有防爆要求,收率也较高。
具体步骤如下:将按1.5:1(摩尔比)混合的干燥氟化钾和氯乙酰胺放入球磨机中球磨半小时,然后将其加入预热好的卧式氟化釜中,在减压条件下加热反应。最终得到含量为95%的氟乙酰胺,收率在72-75%之间。
合成制备方法同样地,氟乙酰胺由氯乙酰胺通过氟化而得。该过程可以在二甲苯或四氯乙烯等溶剂中进行,也可以使用干法(无溶剂法),即干燥的氯乙酰胺与KF加热反应。采用干法则更为简便且没有防爆要求,同时收率也较高。
具体步骤包括:将按1.5:1(摩尔比)混合的干燥氟化钾和氯乙酰胺放入球磨机中球磨半小时,随后将其加入预热好的卧式氟化釜中,在减压条件下加热反应。最终得到含量为95%的氟乙酰胺,收率在72-75%之间。
用途简介氟乙酰胺广泛用于防治棉花、大豆、高粱、小麦及苹果等作物中的蚜虫、柑桔介壳虫和森林螨类等多种害虫。特别对棉花抗性蚜虫具有显著的杀灭效果,同时也可用作高效杀鼠剂。然而,它会对消化道黏膜产生刺激作用,并导致中毒症状,表现为中枢神经系统的过度兴奋、烦躁不安、肌肉震颤等,部分患者可能出现精神障碍和心肌损害。
自1982年6月5日起,我国已全面禁止使用含氟乙酰胺的农药及杀鼠剂,并停止其登记。早在1976年,我国就明令禁止生产该物质。根据农牧渔业部与卫生部在1982年的《农药安全使用规定》,明确禁止将氟乙酰胺作为灭鼠药销售和使用。
用途氟乙酰胺广泛用于防治棉花、大豆、高粱、小麦及苹果等作物中的蚜虫、柑桔介壳虫和森林螨类等多种害虫。尤其对棉花抗性蚜虫具有显著的杀灭效果,同时也可用作高效杀鼠剂。然而,它会对消化道黏膜产生刺激作用,并导致中毒症状,表现为中枢神经系统的过度兴奋、烦躁不安、肌肉震颤等,部分患者可能出现精神障碍和心肌损害。
自1982年6月5日起,我国已全面禁止使用含氟乙酰胺的农药及杀鼠剂,并停止其登记。早在1976年,我国就明令禁止生产该物质。根据农牧渔业部与卫生部在1982年的《农药安全使用规定》,明确禁止将氟乙酰胺作为灭鼠药销售和使用。
上下游信息
反应信息
-
作为反应物:参考文献:名称:Buckle; Heap; Saunders, Journal of the Chemical Society, 1949, p. 914摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 potassium fluoride 作用下, 生成 氟乙酰胺参考文献:名称:Method of preparing fluoroacetamide摘要:公开号:US02416607A1
-
作为试剂:描述:参考文献:名称:Chung, Won Keun; Chung, Jin Hyun; Watanabe, Kyoichi A., Journal of Heterocyclic Chemistry, 1983, vol. 20, p. 457摘要:DOI:
文献信息
-
One-Step Hydroxy Substitution of 4,4’-Dimethoxybenzhydrol with Amides, Lactams, Carbamates, Ureas and Anilines作者:Catherine Henneuse、Thierry Boxus、Lorenzo Tesolin、Guiseppe Pantano、Jacqueline Marchand-BrynaertDOI:10.1055/s-1996-4230日期:1996.4A series of amides, lactams, carbamates, ureas and anilines, equipped with various functionalities, were readily N-alkylated with the 4,4’-dimethoxybenzhydryl residue by reaction with 4,4’-dimethoxybenzhydrol [bis(4-methoxyphenyl)methanol] in acetic acid, at room temperature, under H2SO4 catalysis.
-
Anti-tumour agents申请人:Imperial Chemical Industries PLC公开号:US04992550A1公开(公告)日:1991-02-12A quinazoline of the formula: ##STR1## wherein R.sup.1 is alkyl, cycloalkyl, alkenyl, alkynyl, alkoxy, alkylthio, aryl, aryloxy, arylalkyl, halogeno, hydroxy, mercapto, pyridylthio, pyrimidinylthio, or substituted alkyl or alkoxy; wherein R.sup.2 is hydrogen, alkyl, alkenyl, alkynyl, substituted alkyl or alkanoyl; wherein Ar is phenylene, naphthylene or heterocyclene which is unsubstituted or bears one or more substituents and wherein R.sup.3 is such that R.sup.3 --NH.sub.2 is an amino acid; or a pharmaceutically-acceptable salt or ester thereof. The compounds possess anti-tumour activity.
-
New growth regulators of corn based on N-mono- and N,N-bis-3-butenyldichloroacetamides作者:Yu. N. Bubnov、Yu. Ya. Spiridonov、N. Yu. KuznetsovDOI:10.1007/s11172-018-2080-0日期:2018.2Allylboration of imines, nitriles (including hydrocyanic acid), amides, lactams, aromatic azaheterocycles (pyridines, isoquinoline, and pyrrole) was used to synthesize a series of mono- and bis-3-butenylamines with different structures, which were converted to dichloroacetamides, new analogs of a known safener (herbicide antidote) Dichlormid successfully used in the cultivation of corn throughout the
-
Method for preparing a fluorinated organic compound申请人:RHODIA OPERATIONS公开号:US20140148603A1公开(公告)日:2014-05-29A method for preparing a fluorinated organic compound (II) from an organic compound (I) comprising at least one nucleofugal group Nu, and also a preparation of different specific organic compounds, in particular a fluoro-methylpyrazole compound. The method comprises: a reaction, in the presence of water, of the organic compound (I) and at least one salt providing at least one fluoride anion; and a replacement of at least one nucleofugal group Nu of the compound (I) with a fluorine atom, in order to obtain the fluorinated organic compound (II).
-
A general synthetic route to pentaamminecobalt(III) complexes of N-bonded amides, ureas, carbamates, sulfinamides, sulfonamides and sulfamate作者:David P. Fairlie、W.Gregory JacksonDOI:10.1016/s0020-1693(00)84828-8日期:1990.9A general synthetic procedure for preparing stable cobalt(III) complexes containing selectively nitrogen-bonded ambidentate molecules e.g. amides, ureas, carbamates, sulfinamides, sulfonamides and sulfamate, is described. The method relies on the superior acidity of the nitrogen-bonded, relative to the oxygen-bonded, isomer and this is attributed to much better resonance stabilization of the anionic
表征谱图
-
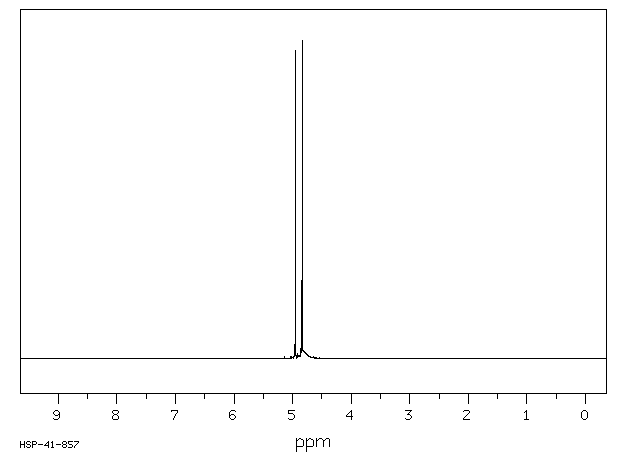
氢谱1HNMR
-
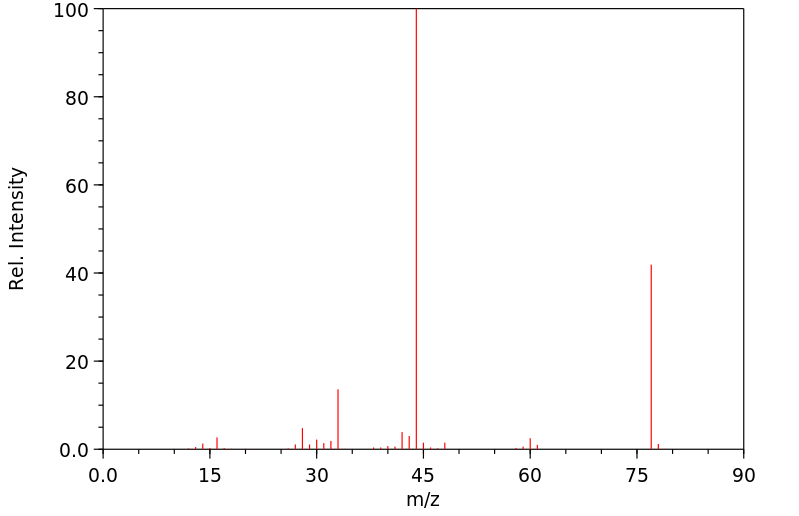
质谱MS
-
碳谱13CNMR
-
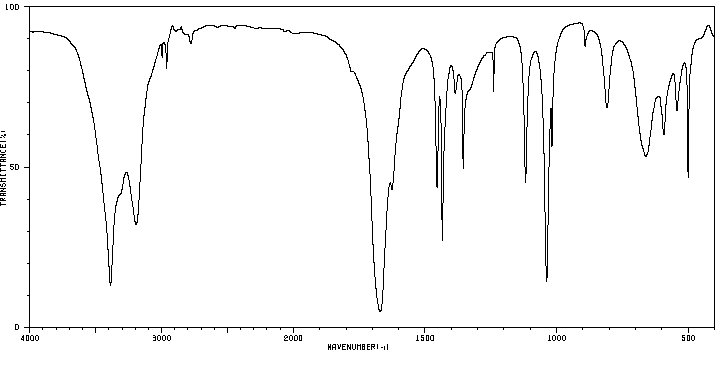
红外IR
-
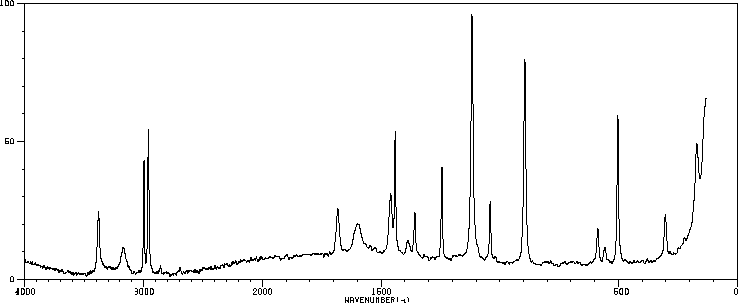
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息