O-乙基硫代乙酸酯 | 926-67-0
中文名称
O-乙基硫代乙酸酯
中文别名
——
英文名称
O-Ethyl thioacetate
英文别名
ethyl thioacetate;Thioacetic acid, O-ethyl ester;O-ethyl ethanethioate
CAS
926-67-0
化学式
C4H8OS
mdl
MFCD08741454
分子量
104.173
InChiKey
IEPFHYMMMGMRNF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:105-110 °C
-
密度:0.9816 g/cm3(Temp: 0 °C)
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:41.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:参考文献:名称:Matsui, Chemisches Zentralblatt, 1909, vol. 80, # II, p. 423摘要:DOI:
-
作为产物:参考文献:名称:Thioimidate N-oxides: nitrones of thio esters摘要:DOI:10.1021/jo00376a028
文献信息
-
Diastereofacial selectivity via aldol reactions using ethyl dithioacetate and ethyl dithiopropionate enolates作者:A.I. Meyers、Robert D. WalkupDOI:10.1016/s0040-4020(01)96754-7日期:1985.1The lithium enolate of ethyl dithioacetate reacts with α-methyl aldehydes to yield the aldol products in which the syn configuration in the positions β and γ to the thiocarbonyl of the product is favored over the anti configuration. This selectivity is solvent-dependent, and is enhanced at lower temperatures. In most cases, syn:anti product ratios obtained under these conditions varied from 57:43 to
-
A Facile and Concise Synthesis of 2-Alkyl- and 2-Aryl-4-oxo- 4<i>H</i>thiopyrano[2,3-<i>b</i>]pyridines作者:Axel Couture、Pierre Grandclaudon、Eric HuguerreDOI:10.1055/s-1989-27288日期:——2-Alkyl- and 2-aryl-4-oxo-4H-thiopyrano[2,3-b]pyridine can be conveniently prepared by reacting the appropriate aromatic and aliphatic O-ethyl thiocarboxylates with the sodium derivative of various alkyl 3-(2-bromopyridyl) ketones.
-
The gas-phase pyrolysis of some primary and secondary thionacetates作者:David B. Bigley、Rosemary E GabbottDOI:10.1039/p29750000317日期:——Alkyl thionacetates undergo thermal elimination by a homogeneous unimolecular mechanism to give alkenes and thioacetic acid as first products. With the primary esters there is an accompanying rearrangement to thiolacetate. A transition state similar to that for acetate pyrolysis is proposed for the elimination reaction, but it is argued that it is more product-like in the thionester case.
-
A New and Concise Synthesis of 3-Aryl- and 3-Alkyl-1<i>H</i>-2-benzothiopyran-1-ones (Thioisocoumarins)作者:Axel Couture、Hélène Cornet、Pierre GrandclaudonDOI:10.1055/s-1990-27113日期:——3-Aryl- and 3-alkyl-1H-2-benzothiopyran-1-ones are readily accessible by reaction of the lithiated N,N-diethyl-o-toluamide (N,N-diethyl-2-methylbenzenecarboxamide) with appropriate aromatic, heteroaromatic and aliphatic thioesters.
-
The influence of substituents on preparation and tautomerism of open-chain β-thioketoesters作者:F. DuusDOI:10.1016/0040-4020(72)88125-0日期:1972.1the synthesis of β-thioketoesters, the acid catalysed reactions of 36 differently substituted β-keto esters with H2S have been studied under various conditions in order to determine the influence of the substituents on reaction course. gem-Dithiols may also be obtained in good yields by this reaction. Treatment of T1(I)-salts of β-thioketo esters with alkyl halides results exclusively in S-alkylation
表征谱图
-
氢谱1HNMR
-
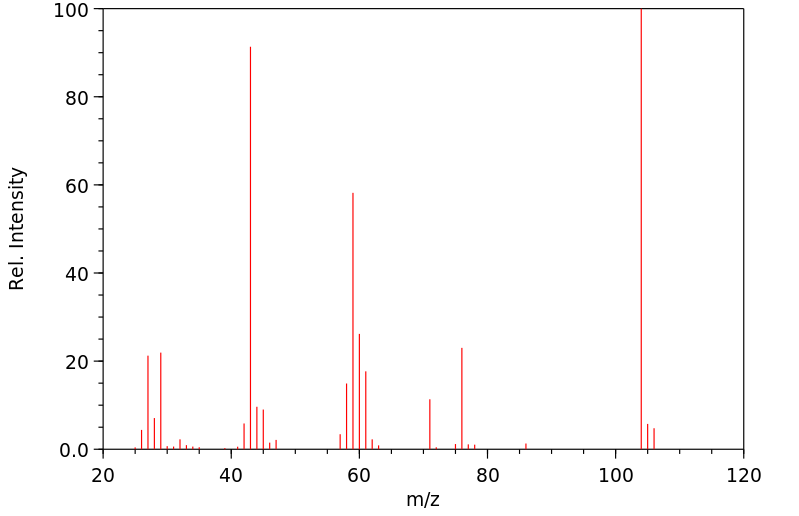
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
羧酸-三聚乙二醇-硫代乙酸酯
磷酸)二氢8-羰基腺苷5'-(
硫代甲酰胺
硫代二乙醇酸二异丙酯
硫代乙酸甲酯
硫代乙酸烯丙酯
硫代乙酸氯代丙酯
硫代乙酸丙酯
硫代乙酸S-正丁酯
硫代乙酸S-乙酯
硫代乙酸S-(2-氨基-乙基)酯盐酸盐
硫代乙酸S-(2,3-二氯丙酯)
硫代乙酸(Z)-S-(3-甲基戊-2-烯-4-炔基)酯
硫代乙酸 S-异丙基酯
硫代乙酸 S-(2-氧代丙基)酯
硫代乙酸 S-(2-氟乙基)酯
硫代丙酸甲酯
硫代丙酸S-乙酯
硫代丙酸S-(2-二甲氨基乙酯)
甲硫代酰胺,N,N-二乙基-
甲基-三聚乙二醇-硫代乙酸酯
环戊硫醇乙酸
环己烷羰基硫代羧酸s-叔丁酯
环己基甲硫醇乙酸
氰甲基硫代乙酸
孟鲁司特钠杂质
叔-丁基-3,6,9,12,15,18,21-七氧杂-34-氧代-33-硫杂三十五烷酸酯
卡托普利杂质6
乙酸3-(乙酰巯基)己酯
乙酰硫酯-六聚乙二醇-炔
乙酰硫酯-八聚乙二醇-炔
乙酰硫酯-三聚乙二醇-炔
乙酰基硫醚
乙酰基硫基-PEG4-炔
乙硫酸,S-环丙基酯
乙硫酸,S-1-环己烯-1-基酯
乙硫酸,S-(3-碘丙基)酯
乙硫酸,S-(1,1-二乙基丙基)酯
乙硫基甲醛
乙基三氟巯基乙酯
丙酸烯丙巯酯
丙酸,3-丙氧基-3-硫代-,乙基酯
s-(2-氨乙基)硫代乙酸
S-(4-氰基丁基)硫代乙酸酯
S-癸基2,2-二甲基硫代丙酸酯
S-甲基环戊烯-1-硫代甲酸酯
S-甲基环己烯-1-硫代甲酸酯
S-甲基氰基硫代乙酸酯
S-甲基2-甲基硫代丙酸酯
S-甲基2-丙氧基硫代丙酸酯