{[(2-甲基-2-丙基)亚磺酰]甲基}苯 | 26756-22-9
中文名称
{[(2-甲基-2-丙基)亚磺酰]甲基}苯
中文别名
——
英文名称
(2-Methyl-propane-2-sulfinylmethyl)-benzene
英文别名
Benzyltert-butylsulfoxide;tert-butylsulfinylmethylbenzene
CAS
26756-22-9
化学式
C11H16OS
mdl
——
分子量
196.313
InChiKey
HOHHGPSMIBONTK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-75 °C
-
沸点:347.6±21.0 °C(Predicted)
-
密度:1.082±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:36.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
上下游信息
反应信息
-
作为反应物:描述:{[(2-甲基-2-丙基)亚磺酰]甲基}苯 在 vanadyl acetylacetonate (S)-2-(N-3',5'-diiodosalicylidene)-amino-3,3-dimethyl-1-butanol 、 双氧水 作用下, 以 二氯甲烷 为溶剂, 生成 叔丁基苄基砜参考文献:名称:钒催化硫氧化中的动力学拆分是获得对映体纯芳基苄基亚砜的有效途径摘要:可以使用钒-席夫碱催化的硫化物氧化和所得亚砜的动力学拆分来制备对映体纯的芳基苄基亚砜。动力学拆分可以与不对称硫化物氧化结合进行,或者从外消旋亚砜开始。DOI:10.1055/s-2006-944187
-
作为产物:描述:参考文献:名称:A Convenient Procedure for the Oxidation of Sterically Hindered Sulfides to Sulfoxides摘要:描述了一种选择性和高效的方法,用于将位阻效应较大的硫化物氧化为亚砜,该方法涉及在硫酸与异戊醇、异丙醇或叔丁醇的催化量存在下,使用过氧化氢和甲醇。DOI:10.1055/s-1990-27059
文献信息
-
Remarkably Mild and Simple Preparation of Sulfenate Anions from β-Sulfinylesters: A New Route to Enantioenriched Sulfoxides作者:Caroline Caupène、Cédric Boudou、Stéphane Perrio、Patrick MetznerDOI:10.1021/jo0478003日期:2005.4.1A general, efficient, and experimentally simple method for the generation of sulfenate salts has been developed using β-sulfinylesters as substrates. The process is based on a retro-Michael reaction, initiated by deprotonation at low temperature. Upon treatment with alkyl halides, the liberated sulfenates are subsequently converted into sulfoxides in good to excellent yield. Extension of the methodology
-
Thermolysis-Induced Two- or Multicomponent Tandem Reactions Involving Isocyanides and Sulfenic-Acid-Generating Sulfoxides: Access to Diverse Sulfur-Containing Functional Scaffolds作者:Shengfeng Wu、Xiaofang Lei、Erkang Fan、Zhihua SunDOI:10.1021/acs.orglett.7b03593日期:2018.2.2Direct reaction of isocyanides with some sulfenic-acid-generating sulfoxides led to the effective formation of the corresponding thiocarbamic acid S-esters in good to high yields. A multicomponent reaction involving isocyanide, sulfoxide, and a suitable nucleophile has also been developed, providing ready access to a diverse range of sulfur-containing compounds, including isothioureas, carbonimidothioic
-
Tungstate-exchanged Mg-Al-LDH catalyst: an eco-compatible route for the oxidation of sulfides in aqueous mediumIICT Communication No: 020222作者:B. M. Choudary、B. Bharathi、Ch. Venkat Reddy、M. Lakshmi KantamDOI:10.1039/b205292j日期:2002.9.11The catalytic oxidation of sulfides selectively to sulfoxides and/or sulfones is realised for the first time with heterogeneous tungstate-exchanged Mg-Al-LDH catalyst using 30% hydrogen peroxide in aqueous media at a faster rate in quantitative yields at room temperature. The heterogeneous catalyst showed higher activity (TOF) over its homogeneous analogues and other heterogeneous catalysts reported so far. The catalyst is well characterised by various instrumental techniques such as FT-IR spectroscopy, thermal analysis (TGA and DTA), powder XRD and chemical analysis. The catalyst is reused for six cycles with consistent activity and selectivity.
-
<i>tert</i>-Butyl Sulfoxides: Key Precursors for Palladium-Catalyzed Arylation of Sulfenate Salts作者:Fabien Gelat、Jean-François Lohier、Annie-Claude Gaumont、Stéphane PerrioDOI:10.1002/adsc.201500368日期:2015.6.15clean generation of sulfenate salts (R1SO−) by pyrolysis of readily available tert‐butyl sulfoxides to give sulfenic acids (R1SOH) and traceless isobutene, followed by hydrogen abstraction with a weak inorganic base (K3PO4). The relevance of this process was exemplified through an in situ palladium‐catalyzed cross‐coupling reaction with aryl halides/triflates leading to aryl sulfoxides. The operationally
-
Asymmetric Synthesis of Aryl Benzyl Sulfoxides by Vanadium-Catalysed Oxidation: A Combination of Enantioselective Sulfide Oxidation and Kinetic Resolution in Sulfoxide Oxidation作者:Pádraig Kelly、Simon E. Lawrence、Anita R. MaguireDOI:10.1002/ejoc.200600320日期:2006.10Enantioselective vanadium-catalysed oxidation of aryl benzyl sulfides using Bolm’s procedure is accompanied by kinetic resolution in the oxidation of the resulting sulfoxides which enhances the enantiopurities of the sulfoxides recovered (typically >90 % ee), albeit with an associated reduction in yield. The effects of ligand, solvent and reaction conditions are discussed in detail.(© Wiley-VCH Verlag
表征谱图
-
氢谱1HNMR
-
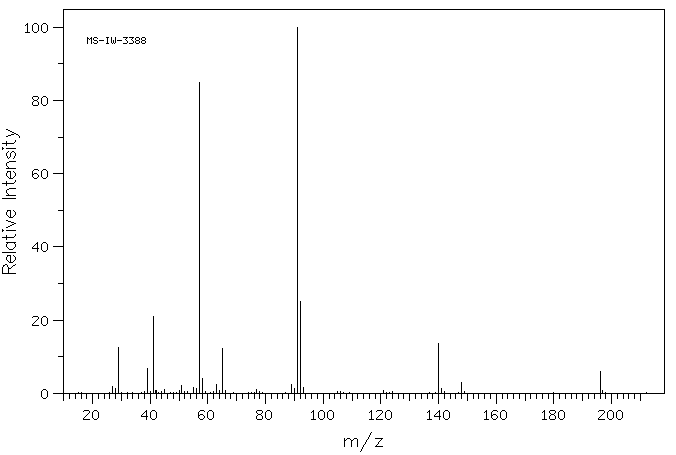
质谱MS
-
碳谱13CNMR
-
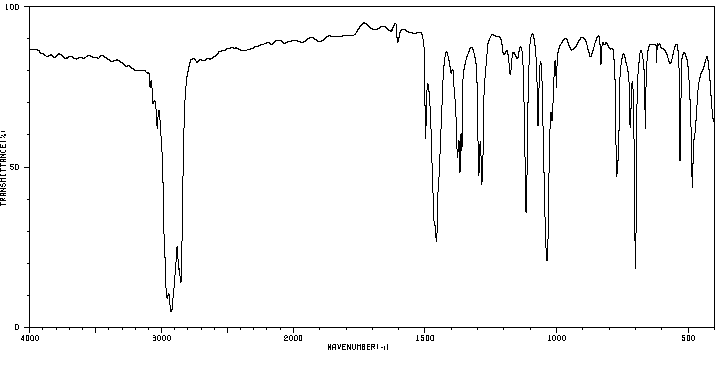
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫