代谢
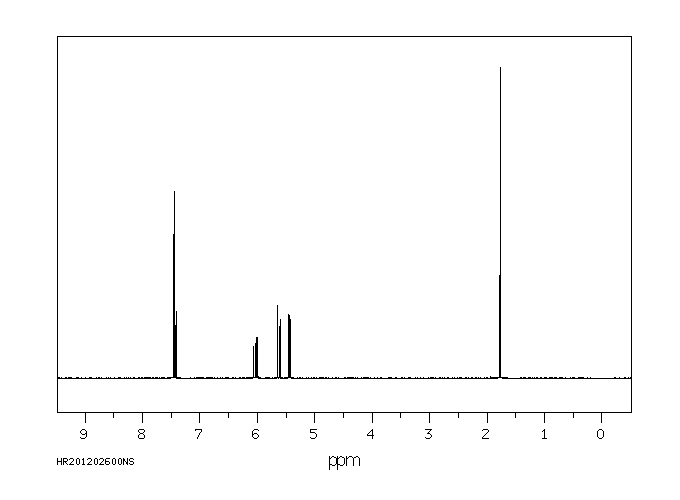
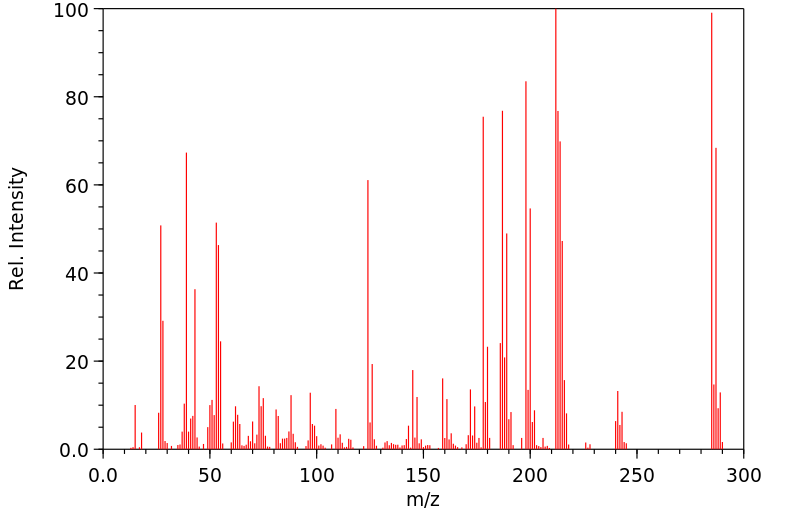
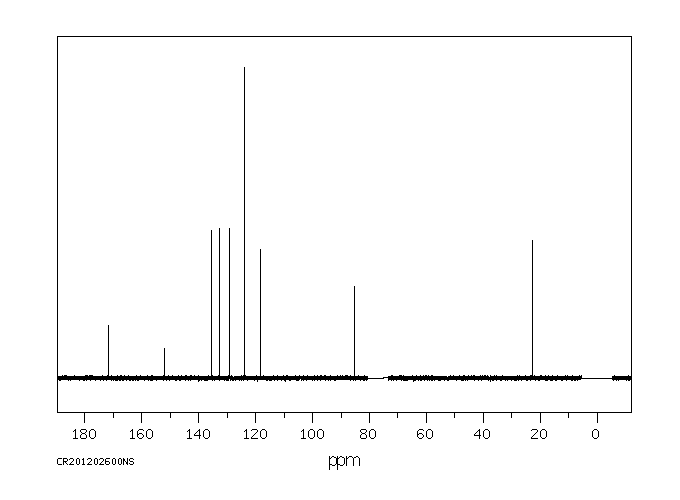
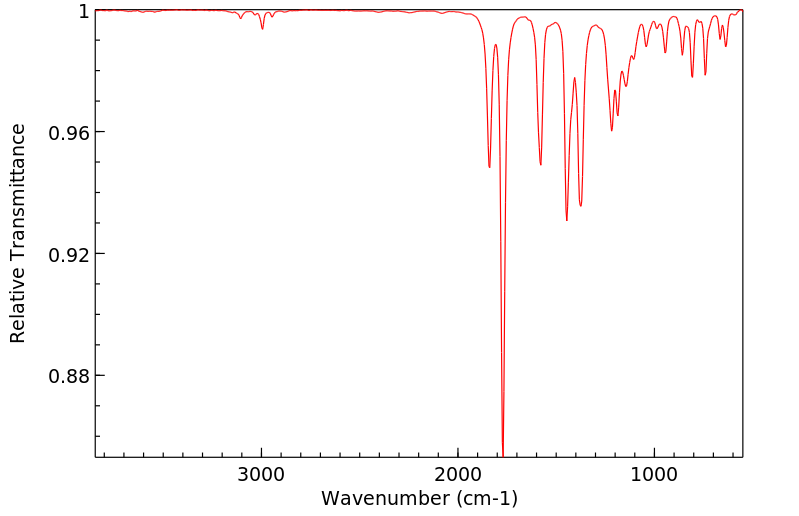
温克洛宗抗雄激素活性的机制是使用重组人雄激素受体(AR)进行研究的。温克洛宗在植物和哺乳动物中的两个主要代谢物是M1(2-[[3,5-二氯苯基)-羧酰胺基]氧基]-2-甲基-3-丁烯酸)和M2(3',5'-二氯-2-羟基-2-甲基丁-3-烯酰胺)。这两种代谢物以剂量依赖性的方式,将雄激素受体靶向细胞核,并抑制由小鼠乳腺肿瘤病毒启动子介导的雄激素诱导的转录激活。3',5'-二氯-2-羟基-2-甲基丁-3-烯酰胺的抑制能力是(2-[[3,5-二氯苯基)-羧酰胺基]氧基]-2-甲基-3-丁烯酸)的50倍,仅比羟基氟他胺低2倍。在二氢睾酮(50 nM)存在的情况下,3',5'-二氯-2-羟基-2-甲基丁-3-烯酰胺(0.2-10 uM)抑制雄激素诱导的雄激素受体与雄激素反应元件DNA的结合。在没有二氢睾酮的情况下,10 uM的3',5'-二氯-2-羟基-2-甲基丁-3-烯酰胺或羟基氟他胺的浓度会促进雄激素受体与雄激素反应元件DNA的结合和转录激活。在LNCaP人前列腺癌细胞内源性的突变雄激素受体(Thr877至Ala)的情况下,3',5'-二氯-2-羟基-2-甲基丁-3-烯酰胺和羟基氟他胺的激动剂活性发生在10倍低于正常浓度的条件下。结果表明,雄激素拮抗剂可以根据配体结合亲和力、浓度和竞争性天然配体的存在而作为激动剂发挥作用。
The mechanism of antiandrogenic activity of vinclozolin was investigated using recombinant human androgen receptor (AR). The two primary metabolites of vinclozolin in plants and mammals are M1 (2-[[3,5-dichlorophenyl)-carbamoyl]oxy]-2-methyl-3-butenoic acid) and M2 (3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide). Both metabolites, in a dose-dependent manner, target androgen receptor to the nucleus and inhibit androgen-induced transactivation mediated by the mouse mammary tumor virus promoter. 3',5'-Dichloro-2-hydroxy-2-methylbut-3-enanilide is a 50-fold more potent inhibitor than (2-[[3,5-dichlorophenyl)-carbamoyl]oxy]-2-methyl-3-butenoic acid) and only 2-fold less than hydroxyflutamide. In the presence of dihydrotestosterone (50 nM), 3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide (0.2-10 uM) inhibits androgen-induced androgen receptor binding to androgen response element DNA. In the absence of dihydrotestosterone, concentrations of 10 uM 3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide or hydroxyflutamide promote androgen receptor binding to androgen response element DNA and activation of transcription. Agonist activities of 3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide and hydroxyflutamide occur at 10-fold lower concentrations with the mutant androgen receptor (Thr877 to Ala) endogenous to LNCaP human prostate cancer cells. The results indicate that androgen antagonists can act as agonists, depending on ligand binding affinity, concentration, and the presence of competing natural ligands.
来源:Hazardous Substances Data Bank (HSDB)