仲丁基 4-羟基苯甲酸 | 17696-61-6
中文名称
仲丁基 4-羟基苯甲酸
中文别名
4-羟基苯甲酸仲丁酯;仲丁基4-羟基苯甲酸;4-羟基苯甲酸1-甲基丙酯
英文名称
sec-butyl 4-hydroxybenzoate
英文别名
sec-butylparaben;4-hydroxy-benzoic acid sec-butyl ester;4-Hydroxy-benzoesaeure-sec-butylester;methylpropyl paraben;4-Hydroxy-benzoesaeure-sek.-butylester;p-Hydroxybenzoesaeure-2-butylester;butan-2-yl 4-hydroxybenzoate
CAS
17696-61-6
化学式
C11H14O3
mdl
MFCD00059626
分子量
194.23
InChiKey
ZUOTXZHOGPQFIU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:60°C
-
沸点:210 °C / 25mmHg
-
密度:1.105
-
闪点:125℃
-
溶解度:可溶于氯仿(少许)、乙酸乙酯(少许)、甲醇(少许)
-
保留指数:1667
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
海关编码:2918290000
SDS
Section I.Chemical Product and Company Identification
Chemical Name 4-Hydroxybenzoic Acid sec-Butyl Ester
Portland OR
Synonym sec-Butyl 4-hydroxybenzoate
Chemical Formula HOC6H4COOCH(CH3)CH2CH3
CAS Number 17696-61-6
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
4-Hydroxybenzoic Acid sec-Butyl Ester 17696-61-6 Min. 98.0 (T) Not available. Not available.
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. IMMEDIATELY flush eyes with runing water for at least 15 minutes. keeping
eyelids open. COLD water may be used. DO NOT use an eye oitment. Flush eyes with running water for a minimum of
15 minutes, occasionally lifting the upper eyelids. Seek medical attention. Treat symptomatically and supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thorough wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. COLD water
may be used. Cover the irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively.
Wash any contaminated clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2).
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
H04e namet)ams (Europe)mpany Identifi
Section VI. Accidental Release Measures
Spill Cleanup Irritating material.
Instructions In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Use a
shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
IRRITANT. Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the
Handling and Storage
container and store in a dry, cool place. Avoid excessive heat and light. DO NOT breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult
a specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Crystalline powder. Not available.
Not available.
Specific Gravity
Molecular Weight 194.23 Partition Coefficient Not available.
Boiling Point Not available. Vapor Pressure Not available.
Melting Point 60°C (140°F) Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye contact. Ingestion. Inhalation.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Continued on Next Page
4-Hydroxybenzoic Acid sec-Butyl Ester
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123
反应信息
-
作为反应物:描述:仲丁基 4-羟基苯甲酸 在 potassium phosphate 、 C50H64Cl2N4O4Rh2 、 potassium carbonate 作用下, 以 甲苯 、 乙腈 为溶剂, 反应 29.0h, 生成 sec-butyl 4-[(triisopropylsilyl)ethynyl]benzoate参考文献:名称:铑催化芳基氨基甲酸酯与炔丙基醇的C-O键炔化摘要:描述了铑催化的芳基氨基甲酸酯与炔丙醇的炔基化反应。这种方法可以通过C–O键活化从氨基甲酸芳基酯提供芳基乙炔。炔丙醇用作炔化剂允许使用与有机金属亲核试剂不相容的多种官能团。该反应还用于扩大氨基甲酸酯部分作为可转化的邻位导向基团的效用。DOI:10.1021/acs.orglett.8b00674
-
作为产物:描述:参考文献:名称:Metabolism and Pharmacokinetics of Oxazaphosphorines摘要:最常用的两种噁唑磷酰胺类药物是环磷酰胺和异环磷酰胺,尽管其他双功能烷化剂仍在研究中。这些药物的药理学特性由其代谢途径决定,因为母体药物相对不活跃。环磷酰胺通过激活生成4-羟基代谢物来消除母体化合物,尽管其他次要的失活途径也起作用。异环磷酰胺通过脱氯乙基化反应更大幅度地失活。更强大的4-羟基代谢物检测方法可能揭示这些药物的临床药理学更多信息,但目前最佳药效学数据显示,母体药物血浆浓度的逆向关系与毒性或抗肿瘤效应相关。环磷酰胺的代谢在应用高剂量化疗时尤为相关。代谢激活途径是可饱和的,因此在高剂量(大于2至4 g/m²)下,药物以失活代谢物的形式排出的比例更大。然而,环磷酰胺和异环磷酰胺都能诱导自身代谢。由于大多数高剂量方案需要连续输注或几天的分次给药,代谢饱和可通过自诱导部分得到补偿。尽管可以对负责激活4-羟基化反应的细胞色素P450同工酶和介导脱氯乙基化反应的同工酶进行定量区分,但临床上尚未证明选择性诱导激活途径或抑制失活途径的能力。描述和预测饱和和自诱导对环磷酰胺净激活相对贡献的数学模型已开发出来。然而,这些模型需要仔细验证,并且可能不适用于它们所衍生的给药方案之外。另一个复杂之处在于这两种药物的手性本质,有迹象表明一种对映体可能比另一种具有更有利的代谢特征。由于其抗肿瘤活性,噁唑磷酰胺类药物在引入临床实践30多年后,仍然是广泛研究的主题。进一步发展分析和分子药理学技术可能进一步优化它们的使用,并允许设计更具选择性的类似物。DOI:10.2165/00003088-200038040-00001
文献信息
-
Precursors for fragrant ketones and fragrant aldehydes申请人:Givaudan SA公开号:EP1262473A1公开(公告)日:2002-12-04The present invention refers to fragrance precursors of formula I for a fragrant ketone of formula II and one or more fragrant aldehydes or ketones of formula III and IV, These fragrance precursors are useful in perfumery, especially in the fine and functional perfumery.
-
[EN] BENZOXAZOLE AND BENZODIAZOLE UV-A SUNSCREENS<br/>[FR] ECRANS SOLAIRES A BASE DE BENZOXAZOLE ET DE BENZODIAZOLE申请人:DSM IP ASSETS BV公开号:WO2004000256A1公开(公告)日:2003-12-31The present invention relates to 1,3- benzoxazole or benzodiazole UV-A sunscreens and to compositions, in particular topical compositions, containing the above UV-A sunscreens.本发明涉及1,3-苯并噁唑或苯并二唑紫外线A防晒剂,以及含有上述紫外线A防晒剂的组合物,特别是含有上述紫外线A防晒剂的局部组合物。
-
A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins作者:Stig D. Friis、Michael T. Pirnot、Lauren N. Dupuis、Stephen L. BuchwaldDOI:10.1002/anie.201703400日期:2017.6.12We report an efficient means of sp2–sp3 cross coupling for a variety of terminal monosubstituted olefins with aryl electrophiles using Pd and CuH catalysis. In addition to its applicability to a range of aryl bromide substrates, this process was also suitable for electron‐deficient aryl chlorides, furnishing higher yields than the corresponding aryl bromides in these cases. The optimized protocol does
-
[EN] IONIC UV-A SUNSCREENS AND COMPOSITIONS CONTAINING THEM<br/>[FR] ECRANS SOLAIRES IONIQUES ANTI-UVA ET COMPOSITIONS CONTENANT CEUX-CI申请人:DSM IP ASSETS BV公开号:WO2005080341A1公开(公告)日:2005-09-01The present invention relates to novel 1,4-dihydropyridine derivatives, to novel cosmetic or dermatological sunscreen compositions containing these derivatives and the use of these derivatives for photoprotecting human skin and/or hair against UV radiation, in particular solar radiation.
-
Ferroelectric liquid crystal polymer申请人:Idemitsu Kosan Co., Ltd.公开号:US04913839A1公开(公告)日:1990-04-03A ferroelectric liquid crystal polymer comprising the recurring units represented by the following general formula: ##STR1## in which k is an integer of from 1 to 30, R.sub.1 is ##STR2## R.sub.2 is --COOR.sub.3, --OCOR.sub.3, --OR.sub.3, or --R.sub.3, in which R.sub.3 is ##STR3## R.sub.4 is --CH.sub.3 or Cl, m is 0 or an integar of from 1 to 10, n is 0 or an integar of from 1 to 10, providing that n is not 0, when R.sub.4 is --CH.sub.3. The ferroelectric liquid crystal polymer of the present invention not only exhibits a ferroelectricity even at temperatures in the vicinity of a room temperature, but also has so fast response speed to the external factors as to be able to display motion pictures, and may be advantageously used as display elements for large size screens and a curved screens.
表征谱图
-
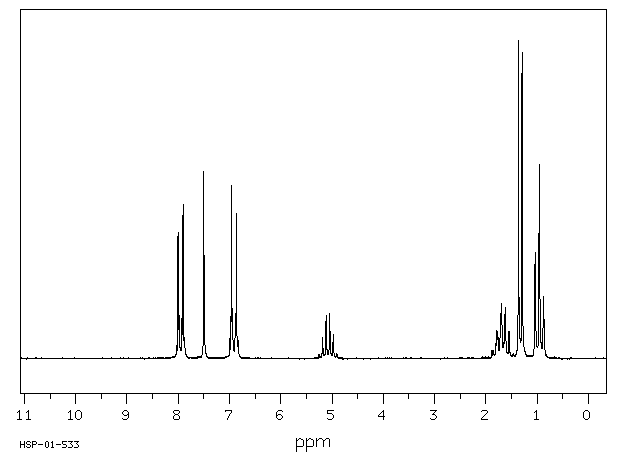
氢谱1HNMR
-
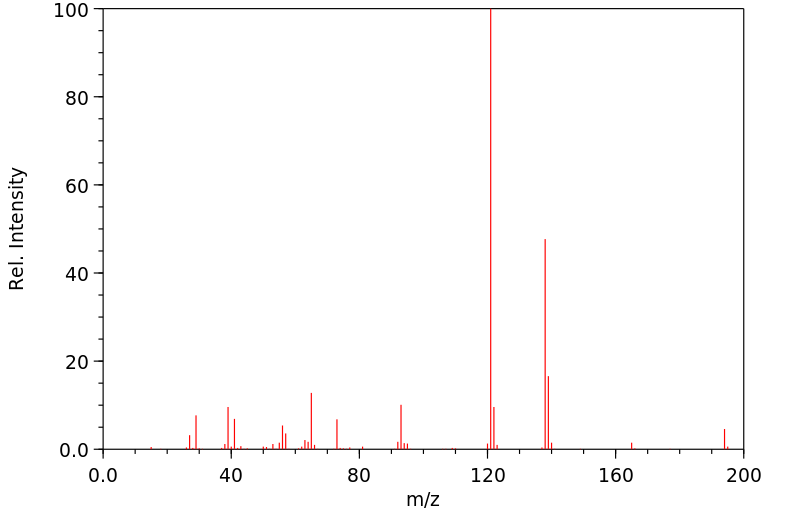
质谱MS
-
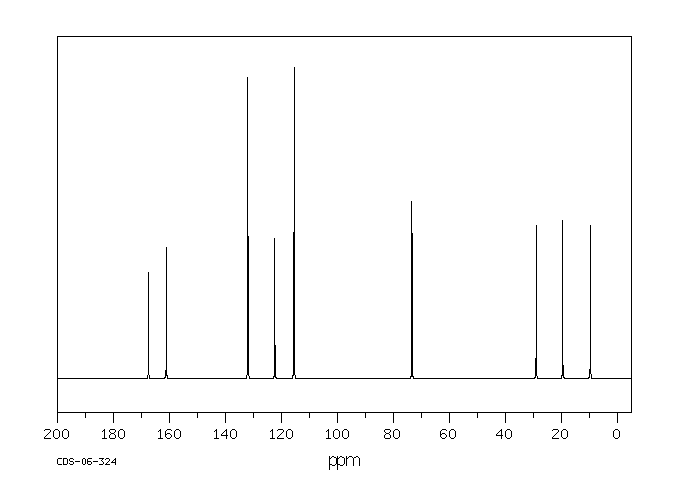
碳谱13CNMR
-
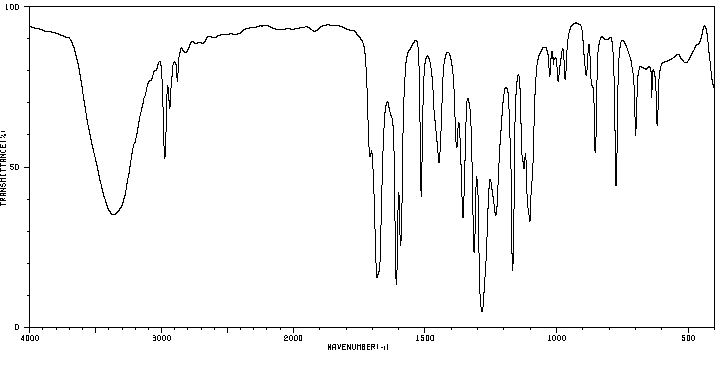
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫