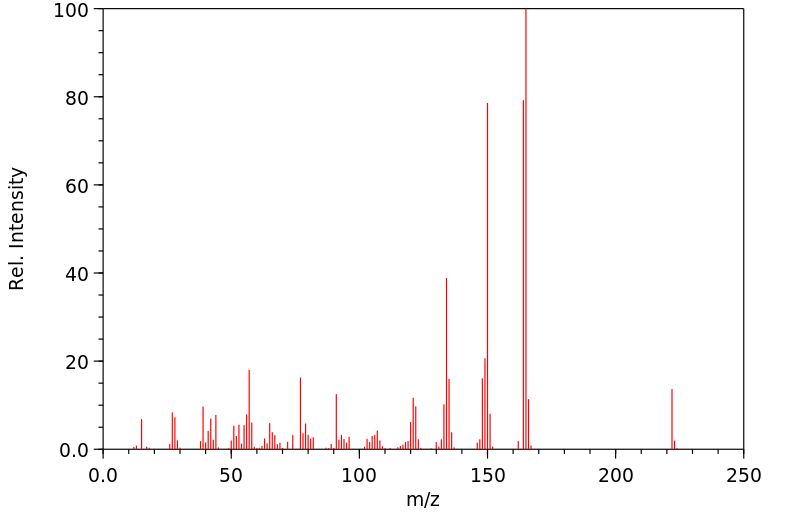
代谢
标记有(14)C的C
MPD在狗体内通过
水解代谢为4-
二甲氨基-3,5-
二甲酚...4-
二甲氨基组的脱甲基作用发生,...4-
氨基组的位移,给出...2,6-二甲基
对苯二酚。
来源:Hazardous Substances Data Bank (HSDB)
代谢
...暴露后...云杉食心虫和烟草食心虫的幼虫将Zectran转化为九种代谢物;家蝇幼虫产生了10种代谢物。在这三种昆虫中发现的四种代谢物被鉴定出来:4-甲基
氨基、4-
氨基、4-甲基甲酰胺和4-甲酰胺-3,5-二甲氧基苄基
甲酸甲酯。
来源:Hazardous Substances Data Bank (HSDB)
代谢
... C
MPD ... 被认为是N-羟甲基Z
ECTRAN /是由云杉和烟草芽虫以及家蝇幼虫在接触Z
ECTRAN后产生的。
来源:Hazardous Substances Data Bank (HSDB)
代谢
... /在人肝脏培养后回收的四种代谢物是/ 4-甲基甲酰胺-3,5-二甲氧基苄基甲基碳酰胺, 4-
氨基-3,5-二甲氧基苄基甲基碳酰胺, 4-甲基
氨基-3,5-二甲氧基苄基甲基碳酰胺, 以及 4-二甲基
氨基-3,5-二甲氧基-N-羟基甲基碳酰胺。还有一些 4-二甲基
氨基-
3,5-二甲氧基苯酚也存在。
来源:Hazardous Substances Data Bank (HSDB)
代谢
甲酰胺通过肝脏酶促
水解;降解产物由肾脏和肝脏排出。
来源:Toxin and Toxin Target Database (T3DB)
毒理性
美沙卡巴酯是一种
胆碱酯酶或
乙酰胆碱酯酶(AChE)
抑制剂。
碳酸酯通过与
胆碱酯酶的活性位点进行
碳酸化作用形成不稳定的复合物。这种抑制作用是可逆的。
胆碱酯酶抑制剂抑制
乙酰胆碱酯酶的作用。由于其基本功能,干扰
乙酰胆碱酯酶作用的
化学物质是强效的神经毒素,即使在低剂量下也会导致过度流涎和流泪。在更高剂量的暴露下,头痛、流涎、恶心、呕吐、腹痛和腹泻通常是显著的症状。
乙酰胆碱酯酶分解神经递质
乙酰胆碱,后者在神经和肌肉接头处释放,以便让肌肉或器官放松。
乙酰胆碱酯酶抑制的结果是
乙酰胆碱积聚并继续发挥作用,使得任何神经冲动持续传递,肌肉收缩不会停止。
来源:Toxin and Toxin Target Database (T3DB)
毒理性
没有关于人类的数据。动物致癌性证据不足。总体评估:第3组:该物质对人类致癌性无法分类。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:泽克特兰
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第12卷:(1976年)一些
氨基甲酸酯、
硫代
氨基甲酸酯和脒基脒
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
Z
ECTRAN在一项实验中被喂给了狗,为期1周,使用的是标记有(14)C的环状物质。实验发现,排出的放射性物质中有3/4出现在尿液中,1/4在粪便中。在尿液中,大约86%的放射性物质以相应的
酚的
葡萄糖苷酸或
硫酸盐形式存在,大约5%是
氢醌,大约2%是未结合的
酚。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
当应用于西兰花时...对
甲醇不溶物的检查表明Zectran已经结合到
木质素中,它可能被永久储存。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在狗身上,口服的Z
ECTRAN剂量主要以4-
二甲氨基-3,5-
二甲酚的形式在尿液中定量排出,主要是作为共轭物,以及2,6-二甲基
对苯醌的共轭形式,还有小量的2,6-二甲基
对苯醌。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
输注Z
ECTRAN...在大鼠中已经观察到胎盘转移;代谢物...也存在于胎儿中。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
当用(14)C标记在羰基位置的Mexacarbate在孕期的第18或19天通过腹腔注射给大鼠时,大部分药物会非常迅速地分布和排泄。注射后15分钟,仅在合并的胎儿中发现了1.7%的放射性,8小时后这一比例降低到了0.8%。根据8小时内从尿液、粪便和呼出气体中回收的总放射性量来计算,孕鼠从Mexacarbate中保留的(14)C比非孕鼠多10.4至23%。
来源:Hazardous Substances Data Bank (HSDB)