(1R,2R)-2-苯甲酰基氨基-环己烷羧酸 | 16524-13-3
中文名称
(1R,2R)-2-苯甲酰基氨基-环己烷羧酸
中文别名
——
英文名称
2-benzoylamino-cyclohexanecarboxylic acid
英文别名
2-Benzamino-hexahydrobenzoesaeure;N-Benzoyl-hexahydroanthranilsaeure;2-Benzamino-cyclohexan-carbonsaeure-(1);2-Benzoylamino-cyclohexancarbonsaeure;2-(Benzoylamino)cyclohexanecarboxylic acid;2-benzamidocyclohexane-1-carboxylic acid
CAS
16524-13-3
化学式
C14H17NO3
mdl
MFCD17020879
分子量
247.294
InChiKey
PUANNVQABXUYKU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.428
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
反应信息
-
作为反应物:描述:4-甲基哌啶 、 (1R,2R)-2-苯甲酰基氨基-环己烷羧酸 以94的产率得到N-[(1R,2S)-2-(4-methylpiperidine-1-carbonyl)cyclohexyl]benzamide参考文献:名称:Cyclohexylbenzamide derivatives, their preparations and their use as摘要:化合物公式为:##STR1## 其中R.sub.1代表能够与芳香环环化的烷氧基;R.sub.2、R.sub.3和R.sub.4各自独立地代表氢、卤素或烷氧基、氨基、烷基氨基或烷基-羰基氨基基团,而Z代表不同取代的哌啶基团;以及它们与药用酸的加合物。公式I的化合物是强效的胃肠运动促进剂。公开号:US05273983A1
-
作为产物:描述:2-benzamidocyclohexane-1-carboxylic acid;1-phenylpropan-1-amine 、 sodium hydroxide 生成 (1R,2R)-2-苯甲酰基氨基-环己烷羧酸参考文献:名称:NOHIRA, HIROYUKI;NOHIRA, MISAKO;YOSHIDA, SHIN-ICHI;OSADA, AYAKO;TERUNUMA,+, BULL. CHEM. SOC. JAP., 61,(1988) N 4, 1395-1396摘要:DOI:
文献信息
-
[EN] FUSED QUINOLINE DERIVATIVE AND USE THEREOF<br/>[FR] DÉRIVÉ DE QUINOLINE FUSIONNÉE ET UTILISATION DE CELUI-CI申请人:TAKEDA PHARMACEUTICAL公开号:WO2005105802A1公开(公告)日:2005-11-10The present invention aims at provision of a quinoline derivative having a neurokinin 2 (NK2) receptor antagonistic action and relates to a compound represented by the formula (I) wherein Rl is a hydrogen atom and the like; R2 is a hydrogen atom, a hydrocarbon group optionally having substituent(s) and the like; R3 is unsubstituted (i.e., absence), a hydrogen atom and the like; R4 and R5 are the same or different and each is a hydrogen atom, a hydrocarbon group optionally having substituent(s), and the like; R6 is (cyclic group optionally having substituent(s)) -carbonyl, and the like; R7, R8, R9 and R10 are the same or different and each is a hydrogen atom, halogen and the like; or R7 and R8, R8 and R9, and R9 and R10 may form a ring together with the adjacent carbon atoms; n is an integer of 1 to 5; --- represents unsubstituted (i.e., absence) or a single bond; and --- represents a single bond or a double bond, or a salt thereof, and the like.
-
1,3,4-Oxadiazoline derivatives and drugs containing these derivatives as active ingredient申请人:——公开号:US20030166574A1公开(公告)日:2003-09-04A 1,3,4-oxadiazoline derivative of formula (I) 1 , wherein W is oxygen, sulfer; R is hydrogen, alkyl, CycA, etc.; AA 1 is a single bond, amino acid residue, etc.; AA 2 is a single bond, amino acid residue, etc.; R 7 and R 8 are hydrogen, alkyl, etc.; R 9 is hydrogen, alkyl, etc., and a non-toxic salt thereof. The compound of formula (I) has an inhibitory activity against cysteine protease and therefore it is useful as an agent for the prophylaxis and/or treatment of inflammatory diseases, diseases induced by apoptosis, diseases induced by disorders of immune responses, autoimmune diseases, diseases induced by decomposition of proteins which compose organism, shock, circulatory system disorders, blood coagulation systems disorders, malignant tumors, acquired immune deficiency syndrome (AIDS) and AIDS-related complex (ARC), parasitic diseases, nerve degeneration diseases, pulmonary disorders, bone resorption diseases, endocrinesthenia, etc.
-
PIPERAZINYL DERIVATIVES AS MODULATORS OF CHEMOKINE RECEPTOR ACTIVITY申请人:Carter Percy H.公开号:US20070179148A1公开(公告)日:2007-08-02The present application describes modulators of MIP-1α of formula (I): or stereoisomers or pharmaceutically acceptable salts thereof, wherein m, T, W, R 1 , R 4 , R 5 , R 5a and R 5b are as defined herein. In addition, methods of treating and preventing inflammatory diseases such as asthma and allergic diseases, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis using said modulators are disclosed.本申请描述了式(I)的MIP-1α的调节剂,或其立体异构体或药学上可接受的盐,其中m、T、W、R1、R4、R5、R5a和R5b如本文所定义。此外,还公开了使用这些调节剂治疗和预防哮喘、过敏病等炎症性疾病,以及类风湿性关节炎和动脉粥样硬化等自身免疫病理的方法。
-
Metallaphotoredox-Catalyzed Cross-Electrophile C<sub>sp</sub><sup>3</sup>–C<sub>sp</sub><sup>3</sup> Coupling of Aliphatic Bromides作者:Russell T. Smith、Xiaheng Zhang、Juan A. Rincón、Javier Agejas、Carlos Mateos、Mario Barberis、Susana García-Cerrada、Oscar de Frutos、David W. C. MacMillanDOI:10.1021/jacs.8b12025日期:2018.12.19pharmaceutically relevant aliphatic structures has been established via metallaphotoredox catalysis. Herein, we report that tris(trimethylsilyl)silanol can be employed as an effective halogen abstraction reagent that, in combination with photoredox and nickel catalysis, allows a generic approach to Csp3-Csp3 cross-electrophile coupling. In this study, we demonstrate that a variety of aliphatic drug-like groups
-
Oxadiazole derivatives and drugs containing these derivatives as the active ingredient申请人:——公开号:US20030166573A1公开(公告)日:2003-09-04An oxadiazole derivative of formula (I) and a non-toxic salt thereof, 1 wherein R is hydrogen, alkyl, CycA, etc.; AA 1 is a single bond, amino acid residue, etc.; AA 2 is a single bond, amino acid residue, etc.; R 7 and R 8 are hydrogen, alkyl, etc.; R 9 is hydrogen, alkyl, etc.; R 10 is hydrogen, alkyl, etc.). The compound of formula (I) has an inhibitory activity against cysteine protease and therefore it is useful as an agent for the prophylaxis and/or treatment of inflammatory diseases, diseases induced by apoptosis, diseases induced by disorders of immune responses, autoimmune diseases, diseases induced by decomposition of proteins which compose organism, shock, circulatory system disorders, blood coagulation system disorders, malignant tumors, acquired immune deficiency syndrome (AIDS) and AIDS-related complex (ARC), parasitic diseases, nerve degeneration diseases, pulmonary disorders, bone resorption diseases, endocrinesthenia, etc.
表征谱图
-
氢谱1HNMR
-
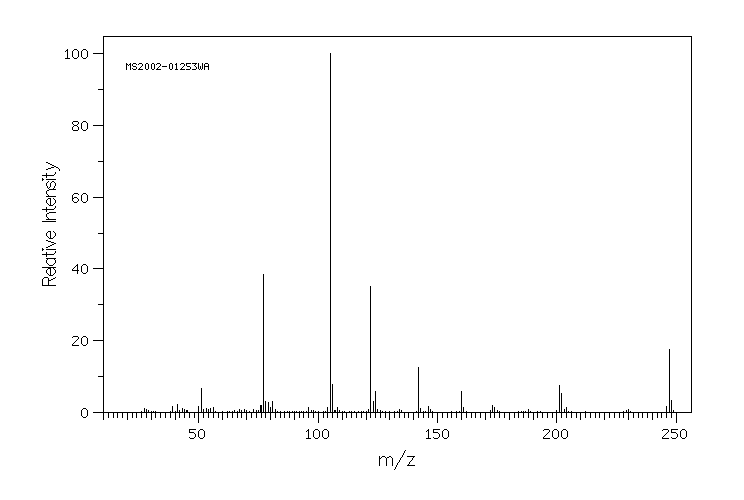
质谱MS
-
碳谱13CNMR
-
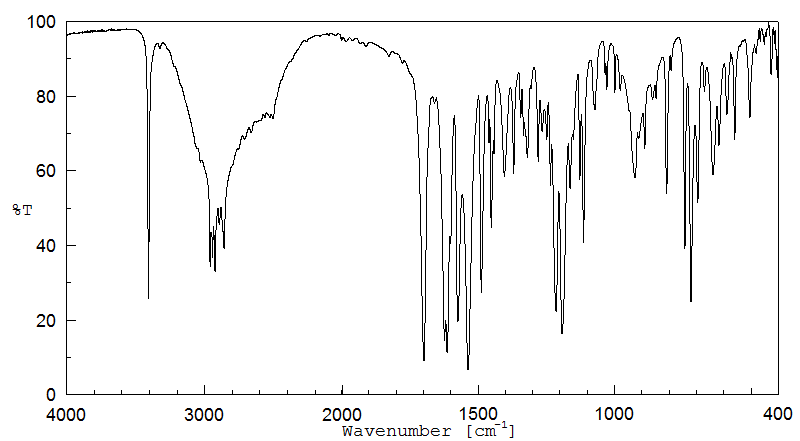
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫