已酸二乙氨基乙醇酯 | 1004-37-1
中文名称
已酸二乙氨基乙醇酯
中文别名
2,6-二甲基吡喃-4(4h)-硫酮
英文名称
2,6-dimethyl-pyran-4-thione
英文别名
2,6-dimethyl-4H-pyran-4-thione;2.6-dimethyl-4-thiopyrone;2,6-Dimethyl-pyran-4-thion;2,6-Dimethylpyran-4-thione
CAS
1004-37-1
化学式
C7H8OS
mdl
MFCD00154298
分子量
140.206
InChiKey
RZGQBLWMLLDDKL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1193
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:41.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2932999099
SDS
反应信息
-
作为反应物:描述:已酸二乙氨基乙醇酯 在 溴代叔丁烷 、 高氯酸四乙基铵 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以75%的产率得到2,2',6,6'-tetramethyl-4,4'-bipyranylidene参考文献:名称:系统性强盗的合成化学双联体摘要:在烷基卤化物的存在下,通过硫代吡喃酮的阴极还原,电化学方法可用于易于合成的供体系统。DOI:10.1016/s0040-4039(00)99837-x
-
作为产物:描述:2,6-dimethyl-4-methoxypyrylium perchlorate 在 sodium sulfide 作用下, 生成 已酸二乙氨基乙醇酯参考文献:名称:Traverso, Annali di Chimica, 1956, vol. 46, p. 821,835摘要:DOI:
文献信息
-
Cephalosporin derivatives申请人:——公开号:US20020049191A1公开(公告)日:2002-04-25The present invention provides a novel series of cephem derivatives of the general formula I 1 wherein R 1 , R 2 , R 3 , R 5 , R 6 , R 7 , A, L 1 , X, n and n′ are as defined herein above. The compounds of formula I are antibacterial agents useful in the treatment of infections in humans and other animals caused by a variety of gram-positive bacteria, particularly methicillin-resistant Staphylococcus aureus . Also included in the invention are processes for preparing the compounds of formula I and pharmaceutical compositions containing said compounds in combination with pharmaceutically acceptable carriers, diluents or excipients.
-
Facile preparation of pyrylium and pyridinium bromides under neutral conditions作者:Eugene A. Cioffi、William F. BaileyDOI:10.1016/s0040-4039(98)00370-0日期:1998.4Pyrylium and pyridinium bromides may be prepared in virtually quantitative yield by reaction of the corresponding base with an excess of tert-butyl bromide under reflux in chloroform solution.
-
Zinc(II) and cadmium(II) halide complexes with 2,6-dimethyl-4H-pyran-4-thione作者:Giuseppina Faraglia、Rodolfo Graziani、Zijian Guo、Umberto Casellato、Sergio SitranDOI:10.1016/s0020-1693(00)83168-0日期:1992.2reflections. The crystal is triclinic, P 1 , with a=7.955(6), b=8.599(6), c=14.098(5) A, α=92.12(3), β=110.96(3) and γ=108.60(3)°. Zn is tetrahedrally coordinated by two Cl and two S(ligand) atoms. Bond distances (mean values) are ZnCl 2.25, ZnS 2.37, SC 1.69 A. The IR absorptions due to metal-halide bond vibrations support a distorted tetrahedral structure for all 1:2 complexes. The [Cd(DMTP)Cl2]n摘要硫代羰基供体2,6-二甲基-4H-吡喃-4-硫酮(DMTP)与通式为[M(DMTP)2X2]和[M(DMTP)X2的卤化锌(II)和镉(II)配合物形成] n(M = Cd或Zn; X =卤化物)。该化合物已经通过IR和质子NMR光谱表征。通过X射线晶体学确定[Zn(DMTP)2Cl2]的晶体结构,并根据2073个观察到的反射将其精炼至最终R为0.060。晶体是三斜晶的,P 1,a = 7.955(6),b = 8.599(6),c = 14.098(5)A,α= 92.12(3),β= 110.96(3)和γ= 108.60(3 )°。Zn由两个Cl和两个S(配体)原子四面体配位。键距(平均值)为ZnCl2.25,ZnS2.37,SC1.69A。由于金属卤化物键振动而产生的IR吸收支持所有1:2配合物的扭曲四面体结构。在八面体环境中,[Cd(DMTP)Cl2] n加合物可能具有
-
New cephem compounds and processes for preparation thereof申请人:FUJISAWA PHARMACEUTICAL CO., LTD.公开号:EP0069872A2公开(公告)日:1983-01-19New cephem compounds of the formula: wherein R1 is amino or a protected amino group; R2 is hydrogen, lower aliphatic hydrocarbon group which may be substituted with suitable substituent(s), cyclo-(lower)alkyl, or cyclo(lower)alkenyl; R3 is lower alkyl which may be substituted with suitable substituents(s); R3a is hydrogen, lower alkyl or amino; and R3b is hydrogen or lower alkyl; and pharmaceutically acceptable salts thereof, and processes for their preparation and a pharmaceutical antibacterial composition comprising them in association with a pharmaceutical antibacterial composition comprising a compound of claim 1 in association with a pharmaceutically acceptable, substantially non-toxic carrier or excipient.
-
Boberg, Friedrich; Nink, Gunter; Bruchmann, Bernd, Phosphorus, Sulfur and Silicon and the Related Elements, 1991, vol. 61, # 12, p. 145 - 150作者:Boberg, Friedrich、Nink, Gunter、Bruchmann, Bernd、Korall, Barbara、Weber, ReglindisDOI:——日期:——
表征谱图
-
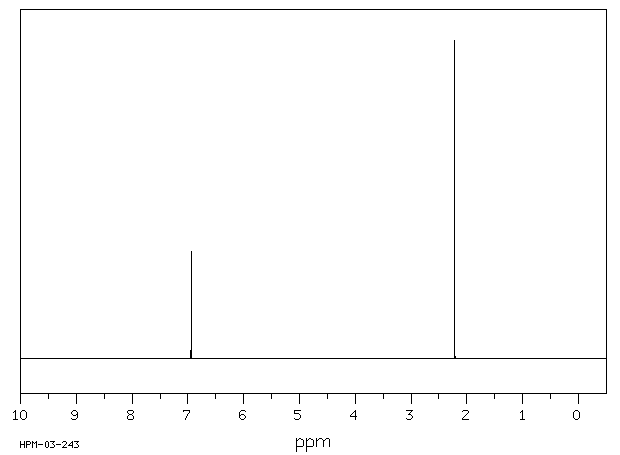
氢谱1HNMR
-
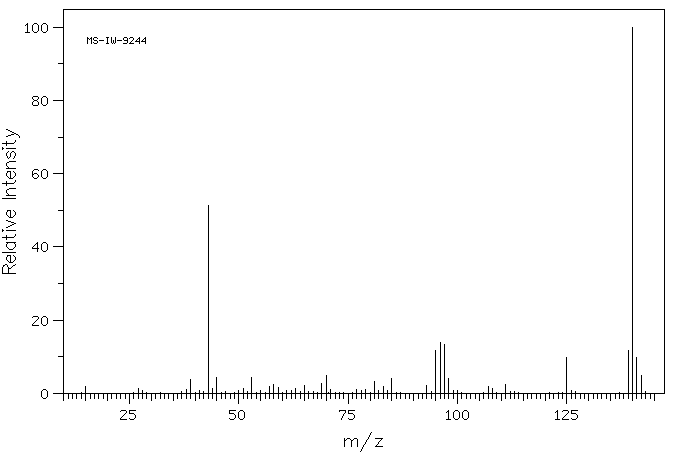
质谱MS
-
碳谱13CNMR
-
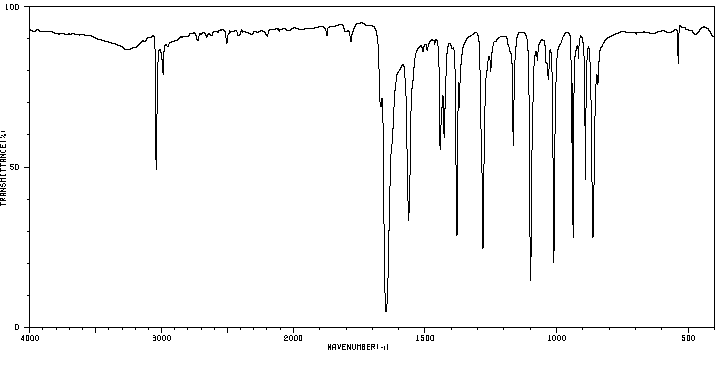
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香薷二醇
顺式-1-(2-呋喃基)-1-戊烯
顺-1,2-二氰基-1,2-双(2,4,5-三甲基-3-噻吩基)乙烯
顺-1,2-(2-噻嗯基)二乙烯
雷尼替丁-N,S-二氧化物
雷尼替丁-N-氧化物
钴(II)双[(2-吡啶基甲基)(叔丁基二甲基甲硅烷基)酰胺]
西拉诺德
螺[环氧乙烷-2,3'-吡咯并[1,2-a]吡嗪]
萘并[2,1,8-def]喹啉
苯硫基溴化镁
苯甲酸,2-[[[7-[[(3.β.)-3-羟基-28-羰基羽扇-20(29)-烯-28-基]amino]庚基]氨基]羰基]
苍术素
羟胺,O-[4-(2-呋喃基)丁基]-
缩水甘油糠醚
紫苏烯
糠醛肟
糠醛氰醇的1-乙氧基乙基醚
糠醇-d2
糠醇
糠基硫醇-d2
糠基硫醇
糠基甲基硫醚
糠基氯
糠基氨基甲酸异丙酯
糠基丙基醚
糠基丙基二硫醚
糠基3-巯基-2-甲基丙酸酯
糠基-异戊基醚
糠基-异丁基醚
糠基 2-甲基-3-呋喃基二硫醚
磷杂茂
碘化N,N,N-三甲基丁烷-1-铵
硫酸异丙基糠酯
硫代磷酸O-糠基O-甲基S-(2-丙炔基)酯
硫代磷酸O-乙基O-糠基S-(2-丙炔基)酯
硫代甲酸S-糠酯
硫代噻吩甲酰基三氟丙酮
硫代乙酸糠酯
硫代丙酸糠酯
硒吩-3-羧酸酰肼
硅烷,三(1-甲基乙基)[(3-甲基-2-呋喃基)氧代]-
硅烷,[2-(3-呋喃基)乙烯基]三甲基-,(E)-
硅烷,(1,1-二甲基乙基)(2-呋喃基甲氧基)二甲基-
砷杂苯
甲酸糠酯
甲氧亚胺基呋喃乙酸铵盐
甲基糠基醚
甲基糠基二硫
甲基呋喃-2-基甲基氨基甲酸酯