代谢
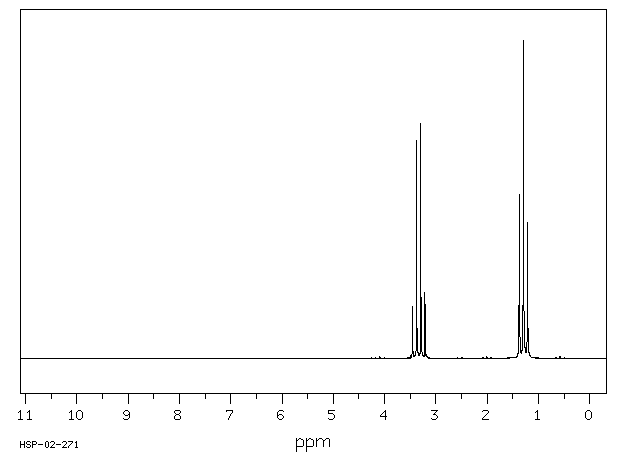
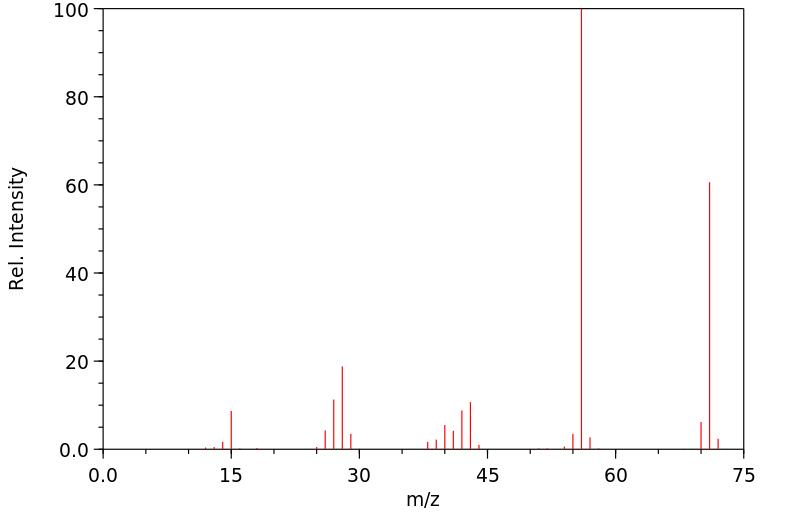
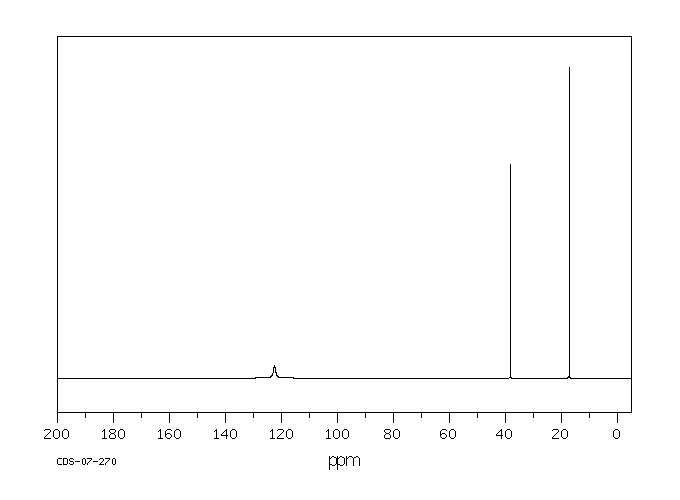
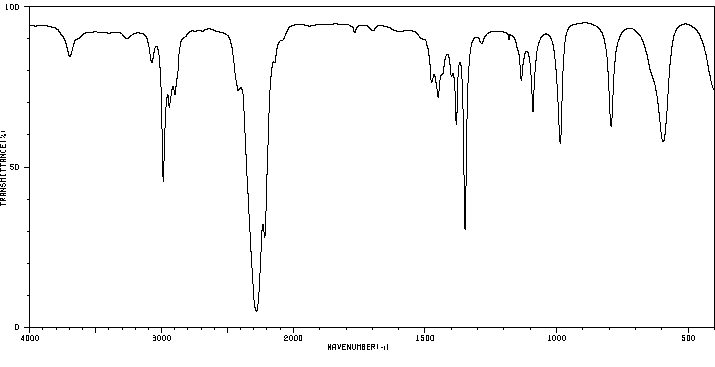
... Reactive isocyanates in general, can react with biological molecules containing amino, alcohol, or sulfhydryl groups, as well as with water. While hydrolysis in an aqueous environment, such as the lung, is theoretically possible, measurements show that alkyl isocyanates are relatively resistant (compared to arylisocyanates) to such hydrolysis.
来源:Hazardous Substances Data Bank (HSDB)