1,1-二乙基烯丙基胺 | 3234-64-8
中文名称
1,1-二乙基烯丙基胺
中文别名
1,1-二乙基炔胺
英文名称
1,1-Diethylpropargylamine
英文别名
3-Ethylpent-1-yn-3-amine
CAS
3234-64-8
化学式
C7H13N
mdl
MFCD00008057
分子量
111.187
InChiKey
WHNOKDNCUNZBLW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:71-72 °C90 mm Hg(lit.)
-
密度:0.828 g/mL at 25 °C(lit.)
-
闪点:70 °F
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3.1
-
危险品标志:F,C
-
安全说明:S16,S26,S36/37/39,S45
-
危险类别码:R11
-
海关编码:2921199090
-
包装等级:II
-
危险类别:3.1
-
危险品运输编号:UN 2733
SDS
| Name: | 1 1-Diethylpropargylamine 97% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 3234-64-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3234-64-8 | 1,1-diethylpropargylamine, 97% | 97 | 221-784-2 |
Risk Phrases: 11 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable. Causes burns.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
Prolonged and/or repeated contact may cause irritation and/or dermatitis. Product tests non-reactive for HBsAg and negative for antibodies to HIV and HCV. However, material should be handled as potentially infectious.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
Prolonged exposure may result in dizziness and general weakness.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes. Flush skin with plenty of soap and water.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Flammable liquid and vapor.
Extinguishing Media:
For small fires, use water spray, dry chemical, carbon dioxide or chemical foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. In case of spill, sweep up solid or absorb liquid with inert material, then place into a biohazard container. Wear protective equipment.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with skin and eyes. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Store in a cool place in the original container and protect from sunlight.
Refrigerator/flammables.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Good general ventilation should be sufficient to control airborne levels. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 3234-64-8: Personal Protective Equipment Eyes: Wear chemical splash goggles. Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin. Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: clear, colorless
Odor: Odorless.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 71.0 - 72.0 deg C @ 90.00mm H
Freezing/Melting Point: 0 deg C
Autoignition Temperature: Not available.
Flash Point: 21 deg C ( 69.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8280g/cm3
Molecular Formula: C7H13N
Molecular Weight: 111.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, none reported, strong oxidants.
Incompatibilities with Other Materials:
None reported.
Hazardous Decomposition Products:
None, irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3234-64-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,1-diethylpropargylamine, 97% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.*
Hazard Class: 3 (8)
UN Number: 2733
Packing Group: II
IMO
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.
Hazard Class: 3.2 (8)
UN Number: 2733
Packing Group: II
RID/ADR
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.
Hazard Class: 3
UN Number: 2733
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F C
Risk Phrases:
R 11 Highly flammable.
R 34 Causes burns.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
S 23 Do not inhale gas/fumes/vapour/spray.
WGK (Water Danger/Protection)
CAS# 3234-64-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3234-64-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3234-64-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-乙基庚-4-炔-3-胺 1,1-Diethyl-1-(pent-2-in)-amin 61822-34-2 C9H17N 139.241 —— 1,1-diethylpropylamine 1571-51-3 C7H17N 115.219
反应信息
-
作为反应物:描述:1,1-二乙基烯丙基胺 在 platinum(IV) oxide copper(I) chloride 、 氢气 、 铜 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 73.0h, 生成 1,1-diethylpropylamine参考文献:名称:Preparation of a series of highly hindered secondary amines, including bis(triethylcarbinyl)amine摘要:DOI:10.1021/jo01311a014
-
作为产物:描述:3-乙基-1-戊炔-3-醇 在 三氯化铁 copper(I) chloride 、 盐酸 、 氨 、 sodium 、 铜 、 calcium chloride 作用下, 以 乙醚 为溶剂, 反应 1.0h, 生成 1,1-二乙基烯丙基胺参考文献:名称:Preparation of a series of highly hindered secondary amines, including bis(triethylcarbinyl)amine摘要:DOI:10.1021/jo01311a014
文献信息
-
Secondary amines申请人:Labaz公开号:US04201725A1公开(公告)日:1980-05-06Methylamine derivatives of the formula: ##STR1## and pharmaceutically acceptable acid addition salts thereof, wherein R.sub.1 and R.sub.3 each represent a hydrogen atom or a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 6 carbon atoms, R.sub.2 represents a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 2 to 7 carbon atoms with the proviso that when R.sub.2 represents an alkenyl radical of the formula CH.dbd.CH--R.sub.6 or an alkynyl radical of the formula C.tbd.C--R.sub.6, in which R.sub.6 represents a hydrogen atom or a straight- or branched-chain alkyl radical of 1 to 5 carbon atoms, R.sub.1 and R.sub.3 each represent an atom of hydrogen or an alkyl radical, R.sub.4 and R.sub.5, which are the same or different, each respresent a hydrogen atom, a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 5 carbon atoms or an .omega.-hydroxyalkyl radical containing from 2 to 5 carbon atoms or R.sub.4 and R.sub.5, when they are taken together represent an alkylene radical containing from 2 to 6 carbon atoms, an alkylidene radical containing from 1 to 5 carbon atoms or the radical --CH.sub.2 --CH.sub.2 --O--CH.sub.2 --CH.sub.2 --, R.sub.1, R.sub.2 and R.sub.3 being such that the trisubstituted methylamine radical possesses no more than 13 carbon atoms. They are useful for treating Parkinson's disease and for correcting extra-pyramidal disturbances provoked by neuroleptics.甲基胺衍生物的公式为:##STR1##以及药学上可接受的酸加成盐,其中R.sub.1和R.sub.3各自代表一个氢原子或一个含有1至6个碳原子的直链或支链烷基、烯基或炔基自由基,R.sub.2代表一个含有2至7个碳原子的直链或支链烷基、烯基或炔基自由基,条件是当R.sub.2代表一个公式为CH.dbd.CH--R.sub.6的烯基自由基或一个公式为C.tbd.C--R.sub.6的炔基自由基时,其中R.sub.6代表一个氢原子或一个含有1至5个碳原子的直链或支链烷基自由基,R.sub.1和R.sub.3各自代表一个氢原子或一个烷基自由基,R.sub.4和R.sub.5相同或不同,各自代表一个氢原子,一个含有1至5个碳原子的直链或支链烷基、烯基或炔基自由基,或一个含有2至5个碳原子的ω-羟基烷基自由基,或者R.sub.4和R.sub.5共同代表一个含有2至6个碳原子的亚烷基自由基,一个含有1至5个碳原子的亚烷基自由基或自由基--CH.sub.2 --CH.sub.2 --O--CH.sub.2 --CH.sub.2 --,R.sub.1、R.sub.2和R.sub.3使得三取代甲基胺自由基最多含有13个碳原子。它们可用于治疗帕金森病,并用于纠正由神经阻滞剂引起的锥体外系扰动。
-
Indolinone derivatives as protein kinase/phosphatase inhibitors申请人:——公开号:US20020052369A1公开(公告)日:2002-05-02The present invention relates to certain 2-indolinone compounds which modulate the activity of protein kinases (“PKs”) and phosphatases. The compounds of this invention are therefore useful in treating disorders related to abnormal PK activity. Pharmaceutical compositions comprising these compounds, methods of treating diseases utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
-
Monoalkylaminomethylation d'organometalliques à l'aide de N-alkyl N-trimethylsilyl alkoxymethylamines作者:G. Courtois、L. MiginiacDOI:10.1016/0022-328x(88)80069-x日期:1988.2The simple or functional N-alkyl-N-trimethylsilylalkoxymethylamines are very convenient new reagents for the route to unsymmetrical secondary functional amines from organometallic compounds (M Al, Mg, Zn) prepared from α-unsaturated halides, functional terminal alkynes or α-bromoesters (or amides).
-
Active derivatives of methylamine in therapeutic compositions and申请人:Labaz公开号:US04057644A1公开(公告)日:1977-11-08Methylamine derivatives of the formula: ##STR1## and pharmaceutically acceptable acid addition salts thereof, wherein R.sub.1 and R.sub.3 each represent a hydrogen atom or a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 6 carbon atoms, R.sub.2 represents a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 2 to 7 carbon atoms with the proviso that when R.sub.2 represents an alkenyl radical of the formula CH.dbd.CH--R.sub.6 or an alkynyl radical of the formula C.tbd.C--R.sub.6, in which R.sub.6 represents a hydrogen atom or a straight- or branched-chain alkyl radical of 1 to 5 carbon atoms, R.sub.1 and R.sub.3 each represent an atom of hydrogen or an alkyl radical, R.sub.4 and R.sub.5, which are the same or different, each represent a hydrogen atom, a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 5 carbon atoms or an .omega.-hydroxyalkyl radical containing from 2 to 5 carbon atoms or R.sub.4 and R.sub.5, when they are taken together represent an alkylene radical containing from 2 to 6 carbon atoms, an alkylidene radical containing from 1 to 5 carbon atoms or the radical --CH.sub.2 --CH.sub.2 --O--CH.sub.2 --CH.sub.2 --, R.sub.1, R.sub.2 and R.sub.3 being such that the trisubstituted methylamine radical possesses no more than 13 carbon atoms. They are useful for treating Parkinson's disease and for correcting extra-pyramidal disturbances provoked by neuroleptics.Methylamine derivatives of the formula: ##STR1## and pharmaceutically acceptable acid addition salts thereof, wherein R.sub.1 and R.sub.3 each represent a hydrogen atom or a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 6 carbon atoms, R.sub.2 represents a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 2 to 7 carbon atoms with the proviso that when R.sub.2 represents an alkenyl radical of the formula CH.dbd.CH--R.sub.6 or an alkynyl radical of the formula C.tbd.C--R.sub.6, in which R.sub.6 represents a hydrogen atom or a straight- or branched-chain alkyl radical of 1 to 5 carbon atoms, R.sub.1 and R.sub.3 each represent an atom of hydrogen or an alkyl radical, R.sub.4 and R.sub.5, which are the same or different, each represent a hydrogen atom, a straight- or branched-chain alkyl, alkenyl or alkynyl radical containing from 1 to 5 carbon atoms or an .omega.-hydroxyalkyl radical containing from 2 to 5 carbon atoms or R.sub.4 and R.sub.5, when they are taken together represent an alkylene radical containing from 2 to 6 carbon atoms, an alkylidene radical containing from 1 to 5 carbon atoms or the radical --CH.sub.2 --CH.sub.2 --O--CH.sub.2 --CH.sub.2 --, R.sub.1, R.sub.2 and R.sub.3 being such that the trisubstituted methylamine radical possesses no more than 13 carbon atoms. They are useful for treating Parkinson's disease and for correcting extra-pyramidal disturbances provoked by neuroleptics.
-
Stille Reactions Catalytic in Tin: A “Sn−F” Route for Intermolecular and Intramolecular Couplings作者:William P. Gallagher、Robert E. MaleczkaDOI:10.1021/jo0484169日期:2005.2.1hypercoordinate by KF(aq) allows Me3SnH to be recycled during a Pd(0)-catalyzed hydrostannation/Stille cascade. Starting with a variety of alkynes, in situ vinyltin formation is followed by Stille reaction with aryl, styryl, benzyl, or vinyl electrophiles present in the reaction mixture. Both inter- and intramolecular versions of the process are possible with tin loads of approximately 6 mol %. Regeneration
表征谱图
-
氢谱1HNMR
-
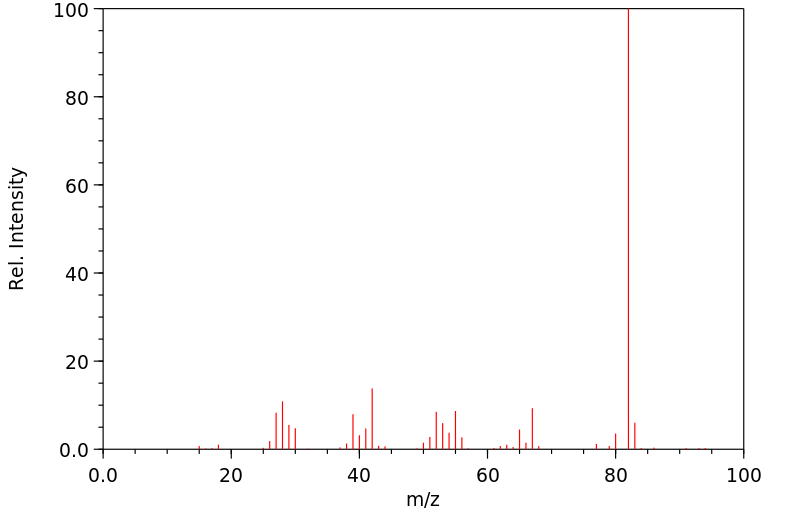
质谱MS
-
碳谱13CNMR
-
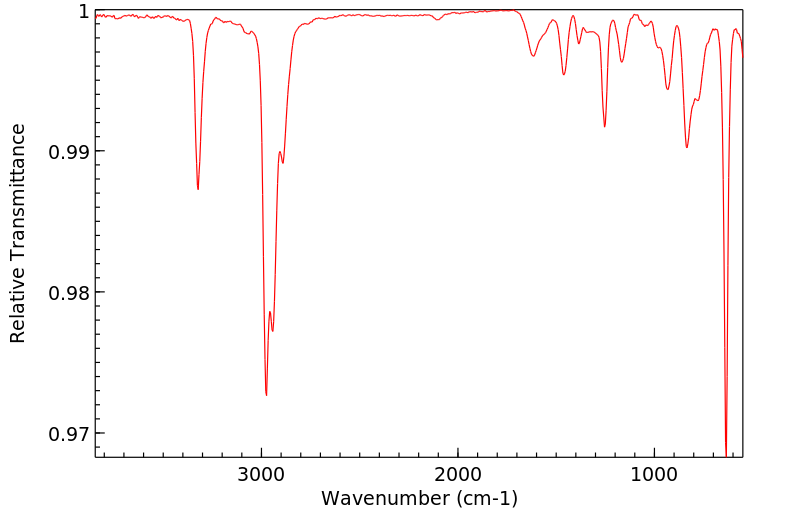
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锗烷,三甲基[3-(三甲基甲锡烷基)-2-炔丙基]-
锗烷,三甲基-2-炔丙基-
铜,1-戊炔基-
甲基炔丙基硫化物
甲基乙炔和丙二烯混合物
甲基丙-2-炔基氰基二硫代亚氨酸酯
甲基-D3-乙炔
环戊基乙炔
环己基乙炔
环丙乙炔
炔丙胺
炔丙基膦
炔丙基碘化物
炔丙基叔丁基二甲基硅烷
炔丙基三甲基硅烷
炔丙基三乙基硅烷
氘乙炔
戊-1-炔-3-胺
戊-1,3-二炔
戊-1,2-二烯-4-炔
异氰基-乙炔
己基(己-5-炔基)甲基硅烷
己-1-炔银
四碳化铀
反式-4-(2-丙炔基)-环己烷甲醇
双(三甲基锡)乙炔
双(三氟甲基)锌
十四碳-1,4-二炔
十四碳-1,3-二炔
十八碳-1,17-二炔
十八炔
十三碳-1,7-二炔
十三碳-1,12-二炔
十一碳-1,5-二炔
亚硫酸二(2-丙炔基)酯
二甲基炔丙基溴化硫
二炔丙基硫醚
二乙炔基-二甲基-锗烷
二丙-1-炔基汞
二[2-甲氧基乙基汞(II)]乙炔
二(三正丁基甲锡烷基)乙炔
二(3-羟基-1-丙炔基)汞(II)
乙炔锂乙二胺配合物
乙炔银
乙炔基环己烷钠
乙炔基环丙烷氯化镁
乙炔基(三甲基)锗烷
乙炔基(三甲基)硅烷铜(1+)
乙炔基(三甲基)硅烷溴化镁
乙炔基(三甲基)硅烷氯化镁