1,1-二甲基-2-丙烯基乙酸酯 | 24509-88-4
中文名称
1,1-二甲基-2-丙烯基乙酸酯
中文别名
——
英文名称
2-methyl-3-buten-2-yl acetate
英文别名
2-methylbut-3-en-2-yl acetate;1,1-dimethylallyl acetate;3-Buten-2-ol, 2-methyl-, acetate
CAS
24509-88-4
化学式
C7H12O2
mdl
——
分子量
128.171
InChiKey
ZJVWGOLNVKJRDF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:120-122 °C
-
密度:0.8940 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915390090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:铜催化的烯烃,亚硝基芳烃和N-羟基烯丙胺的三组分环形成熔融的恶嗪烷/异恶唑烷杂环摘要:使用CuCl / O 2催化剂可实现亚硝基芳烃,烯烃和N-羟基烯丙胺之间的一锅级联反应,形成具有出色的非对映选择性(dr> 20:1)的稠合的恶嗪烷/异恶唑烷杂环。为了增强合成效用,我们开发了所得杂环的两个N-O键的连续裂解。推测了涉及硝酮中间体与它们的链状烯烃的偶极[3 + 2]环加成的机理以形成这些杂环。DOI:10.1002/anie.201611388
-
作为产物:描述:1,1-二甲基丙-2-炔基乙酸酯 在 palladium-barium carbonate 、 Petroleum ether 作用下, 生成 1,1-二甲基-2-丙烯基乙酸酯参考文献:名称:The Controlled Semi-Hydrogenation of tert-Ethynyl Carbinols and Their Acetate Esters1摘要:DOI:10.1021/jo01116a021
-
作为试剂:描述:参考文献:名称:Nesmejanow et al., Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1949, p. 623,627136,138摘要:DOI:
文献信息
-
Fluoroboric acid adsorbed on silica gel as a new and efficient catalyst for acylation of phenols, thiols, alcohols, and amines作者:Asit K. Chakraborti、Rajesh GulhaneDOI:10.1016/s0040-4039(03)00683-x日期:2003.4Fluoroboric acid supported on silica gel efficiently catalyzes acylation of structurally diverse phenols, alcohols, thiols, and amines under solvent free conditions. Acid-sensitive alcohols are smoothly acylated without competitive side reactions.
-
Hydroxyl-Directed Stereoselective Diboration of Alkenes作者:Thomas P. Blaisdell、Thomas C. Caya、Liang Zhang、Amparo Sanz-Marco、James P. MorkenDOI:10.1021/ja504228p日期:2014.7.2An alkoxide-catalyzed directed diboration of alkenyl alcohols is described. This reaction occurs in a stereoselective fashion and is demonstrated with cyclic and acyclic homoallylic and bishomoallylic alcohol substrates. After oxidation, the reaction generates 1,2-diols such that the process represents a method for the stereoselective directed dihydroxylation of alkenes.
-
Palladium-Catalyzed γ-Selective and Stereospecific Allyl−Aryl Coupling between Acyclic Allylic Esters and Arylboronic Acids作者:Hirohisa Ohmiya、Yusuke Makida、Dong Li、Masahito Tanabe、Masaya SawamuraDOI:10.1021/ja9092264日期:2010.1.20Reactions between acyclic (E)-allylic acetates and arylboronic acids in the presence of a palladium catalyst prepared from Pd(OAc)(2), phenanthroline (or bipyridine), and AgSbF(6) (1:1.2:1) proceeded with excellent gamma-selectivity to afford allyl-aryl coupling products with E-configuration. The reactions of alpha-chiral allylic acetates took place with excellent alpha-to-gamma chirality transfer在由 Pd(OAc)(2)、菲咯啉(或联吡啶)和 AgSbF(6) (1:1.2:1) 制备的钯催化剂存在下,无环 (E)-烯丙基乙酸酯和芳基硼酸之间的反应进行得非常好γ-选择性提供具有 E-构型的烯丙基-芳基偶联产物。α-手性烯丙基乙酸酯的反应发生了具有顺式立体化学的优异的 α 到 γ 手性转移,以产生在苄基位置具有立体中心的烯丙基化芳烃。该反应在烯丙基乙酸酯和芳基硼酸中都可以耐受范围广泛的官能团。此外,肉桂醇衍生物的γ-芳基化得到含有未共轭烯基取代基的墒二芳基烷烃衍生物。该方法的合成效用通过其在 (+)-舍曲林(一种抗抑郁药)的有效合成中的应用得到证明。观察到的 γ-区域选择性和 E-1,3-syn 立体化学基于 Pd(II) 机制进行合理化,该机制涉及阳离子单(酰氧基)钯(II)配合物和芳基硼酸之间的金属转移,以及定向碳钯化,然后是 Syn-β -酰氧基消除。与可能的中间体相
-
Alkylation des ions carboxylates par les sels de sulfonium: influence des sels de cuivre作者:B. Badet、M. Julia、M. Ramirez-Munoz、C.A. SarrazinDOI:10.1016/s0040-4020(01)91553-4日期:1983.1Sulfonium salts are extremely powerful alkylating agents, particularly in the two-phase technique both solid-liquid and liquid-liquid). Alkylation of carboxylate salts by sulfonium salts does not show very large dependence on ratio of reactivities of various groups attached to the sulfur and mixtures of esters are often obtained. In the presence of copper(I) salts, there is a strong acceleration of
-
Indium(III) chloride as a new, highly efficient, and versatile catalyst for acylation of phenols, thiols, alcohols, and amines作者:Asit K. Chakraborti、Rajesh GulhaneDOI:10.1016/s0040-4039(03)01641-1日期:2003.8Indium(III) chloride efficiently catalyses the acylation of structurally diverse phenols, alcohols, thiols, and amines under solvent free conditions. Acid sensitive alcohols are smoothly acylated without competitive side reactions. Acylation of 2-hydroxynaphthalene is carried out with carboxylic acids adopting the mixed anhydride protocol using trifluoroacetic anhydride.
表征谱图
-
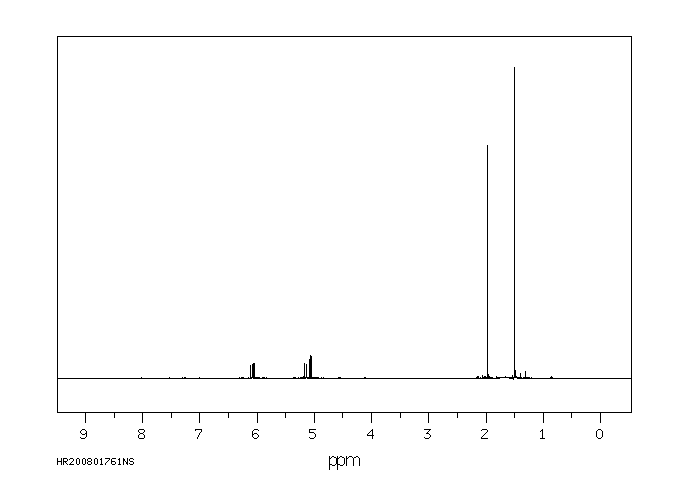
氢谱1HNMR
-
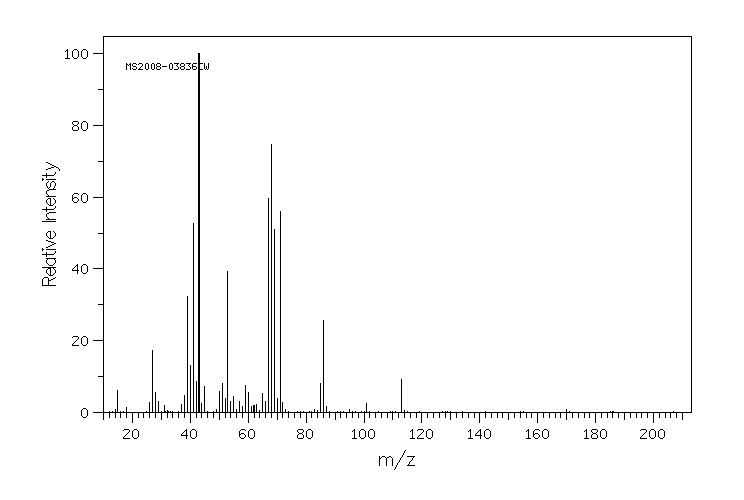
质谱MS
-
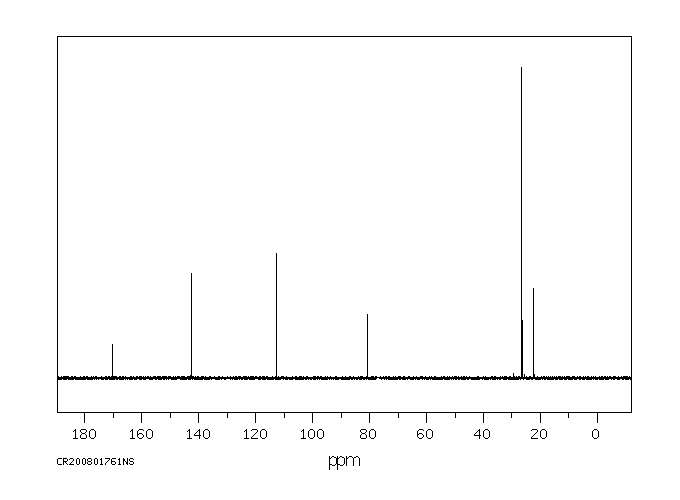
碳谱13CNMR
-
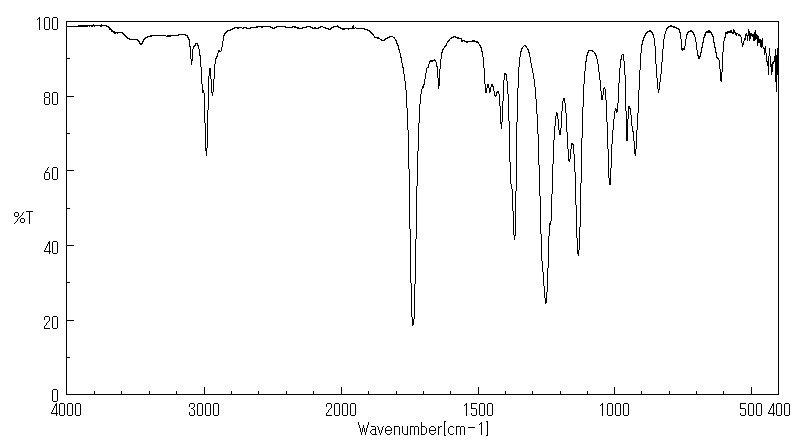
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸