抗蚜威 | 23103-98-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90.5°C
-
沸点:380.88°C (rough estimate)
-
密度:1.1387 (rough estimate)
-
闪点:>100 °C
-
溶解度:可溶于丙酮(少许)、氯仿(少许)、甲醇(少许)
-
LogP:1.700
-
颜色/状态:Colorless solid
-
蒸汽压力:7.28X10-6 mm Hg @ 25 °C
-
稳定性/保质期:
避免接触强氧化剂和光线。
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa = 4.53
-
碰撞截面:154.68 Ų [M+H]+ [CCS Type: TW]
-
保留指数:1796;1807;1810;1816;1816;1816.2;1810.3;1813;1803.1;1817;1818.7;1826.1;1819.3
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:17
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.55
-
拓扑面积:58.6
-
氢给体数:0
-
氢受体数:5
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:T,N
-
安全说明:S16,S22,S26,S36,S37,S45,S60,S61
-
危险类别码:R25,R50/53
-
WGK Germany:3
-
危险品运输编号:UN 2811
-
RTECS号:EZ9100000
-
海关编码:2933599015
-
包装等级:III
-
危险类别:6.1(b)
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 抗蚜威;5,6-二甲基-2-二甲氨基-4-嘧啶基二甲基氨基甲酸酯 |
| 化学品英文名称: | Pirimicarb;Aphox |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 23103-98-2 |
| 分子式: | C 11 H 18 N 4 O 2 |
| 分子量: | 238.33 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:抗蚜威;5,6-二甲基-2-二甲氨基-4-嘧啶基二甲基氨基甲酸酯 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第6.1类 毒害品 |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 摄入会中毒。对人有致突变作用。受热分解释出氮氧化物。 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解,放出有毒的烟气。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 泡沫、干粉、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好口罩、护目镜,穿工作服。不要直接接触泄漏物,小心扫起,装入备用袋中。被污染地面用肥皂或洗涤剂刷洗,经稀释的污水放入废水系统。无法利用的应将其倒至空旷地方深埋。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:0.2mg/m3 美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,加强通风。 |
| 呼吸系统防护: | 作业工人应该佩戴防毒口罩。必要时佩戴自给式呼吸器。 |
| 眼睛防护: | 必要时戴化学安全防护眼镜。 |
| 身体防护: | 穿相应的防护服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 白色无臭结晶体。 |
| pH: | |
| 熔点(℃): | 90.5 |
| 沸点(℃): | |
| 相对密度(水=1): | |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | 0.399*(10-5)/30℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 11 H 18 N 4 O 2 |
| 分子量: | 238.33 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,易溶于多数有机溶剂。 |
| 主要用途: | 用作农用杀虫剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | 光照可分解。 |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氮氧化物。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:147mg/kg(大鼠经口)107mg/kg(小鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61888 |
| UN编号: | 2757 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。避光保存。防止受潮和雨淋。防止阳光曝晒。专人保管。应与氧化剂、食用化工原料分开存放。不能与粮食、食物、种子、饲料、各种日用品混装、混运。操作现场不 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
大鼠急性经口LD₅₀为68~147mg/kg,小鼠为107mg/kg。大鼠急性经皮LD₅₀大于500mg/kg。2年慢性毒性试验表明,大鼠无作用剂量为每天12.5mg/kg,狗为1.8mg/kg。对动物无致畸、致癌、致突变作用。在三代繁殖和神经毒性试验中未见异常情况。多数鱼类LC₅₀为32~40mg/kg,对蜜蜂安全。
化学性质抗蚜威是一种白色无臭结晶体,熔点90.5℃,蒸气压4×10⁻³Pa(30℃)。它能溶于醇、酮、酯、芳烃、氯化烃等多种有机溶剂:甲醇23g/100mL,乙醇25g/100mL,丙酮40g/100mL;难溶于水(0.27g/100mL)。遇强酸、强碱或紫外光照射易分解。在一般条件下贮存较稳定,对一般金属设备不腐蚀。
用途抗蚜威是一种高效、选择性杀蚜虫剂,具有触杀、熏蒸、内吸作用,并且对叶面有渗透性。用于防治粮食、果树、蔬菜、花卉上的蚜虫,如防治甘蓝、白菜、豆类、烟草、麻苗上的蚜虫,用50%可湿性粉剂2000-4000倍喷雾。抗蚜威对棉蚜基本无效。
它也是一种高效专一杀螨剂,具有触杀、熏杀、内吸渗透作用,对有机磷产生抗生的蚜虫仍有杀灭作用。
生产方法由不对称二甲基胍硫酸盐与α-甲基乙酰乙酸乙酯于60℃反应2小时,环合成2-(N,N-二甲氨基)-5,6-二甲基-4-羟基嘧啶,然后在苯中于低温下与光气反应,最后与二甲胺反应得到抗蚜威。
制备方法一- N,N-二甲基胍的制备:由硫脲与硫酸二甲酯作用,再同二甲胺或二甲胺盐酸盐反应制得。
- α-甲基乙酰乙酸乙酯的制备:将乙酰乙酸乙酯用硫酸二甲酯或碘甲烷甲基化,制得α-甲基乙酰乙酸乙酯。
- 2-二甲基氨基-5,6-二甲基-4-羟基嘧啶的制备:将14g N,N-二甲基胍(硫酸盐)和4.6g 缚酸剂、醇混合后搅拌2小时,加入α-甲基乙酰乙酸乙酯和溶剂,搅拌下慢慢升温,在140℃左右反应2小时,减压回收溶剂,固体用氯仿溶解并调节pH值至7,氯仿浓缩至干,得产品15.7g,含量92.69%,收率93.43%。
- 抗蚜威的合成:采用氨基甲酸酯类农药生产方法。
在含35mL苯溶液中加入7.1g 5,6-二甲基-2-二甲氨基-4-羟基嘧啶和4.3g三乙胺,于5~8℃反应45分钟。滴加10mL 含3.9g 二甲胺的水溶液,并搅拌至反应温度小于10℃。在10℃下继续搅拌超过30分钟,加入50mL 水至固体全部溶解。分出苯相并用水洗、饱和Na₂CO₃溶液处理即可得抗蚜威。熔点88~90℃。
类别农药
毒性分级高毒
急性毒性口服-大鼠 LD₅₀: 100毫克/公斤;口服-小鼠 LD₅₀: 107毫克/公斤
可燃性危险特性燃烧产生有毒氮氧化物气体
储运特性库房通风低温干燥;与食品原料分开储运
灭火剂干粉、泡沫、砂土
职业标准STEL 0.05 毫克/立方米
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 脱甲基-抗蚜威 2-(methylamino)-5,6 dimethylpyrimidin-4-yl dimethylcarbamate 30614-22-3 C10H16N4O2 224.263 5,6-二甲基-2-(N-甲酰基甲氨基)-4-嘧啶基二甲基氨基甲酸酯 2-<(methylformyl)amino>-5,6-dimethylpyrimidin-4-yl dimethylcarbamate 27218-04-8 C11H16N4O3 252.273 —— 2-(formylamino)-5,6-dimethylpyrimidin-4-yl dimethylcarbamate 178273-91-1 C10H14N4O3 238.246 —— 2-(amino)-5,6-dimethylpyrimidin-4-yldimethylcarbamate 30614-21-2 C9H14N4O2 210.236 2-二甲胺基-4-羟基-5,6-二甲基嘧啶 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine 40778-16-3 C8H13N3O 167.211
反应信息
-
作为反应物:描述:参考文献:名称:Environmental application of an industrial waste as catalyst for the electro-Fenton-like treatment of organic pollutants摘要:酸性矿山排水污泥(AMDS)作为含有高金属含量的工业废物,在处理有机污染废水方面作为电-Fenton类技术的催化剂的应用已经被研究。DOI:10.1039/c4ra15934a
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
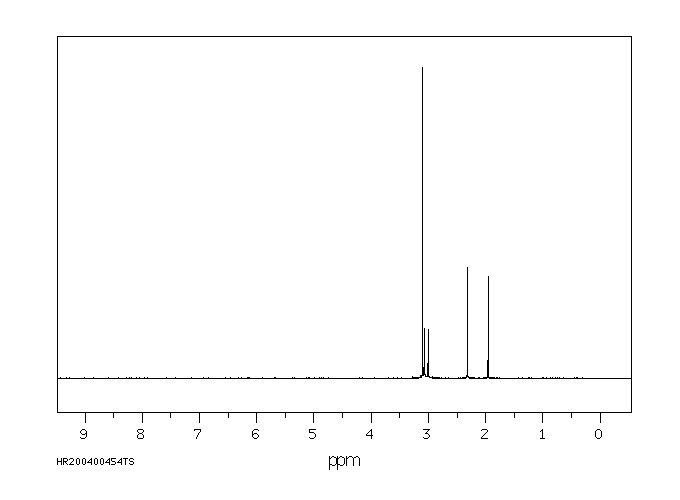
氢谱1HNMR
-
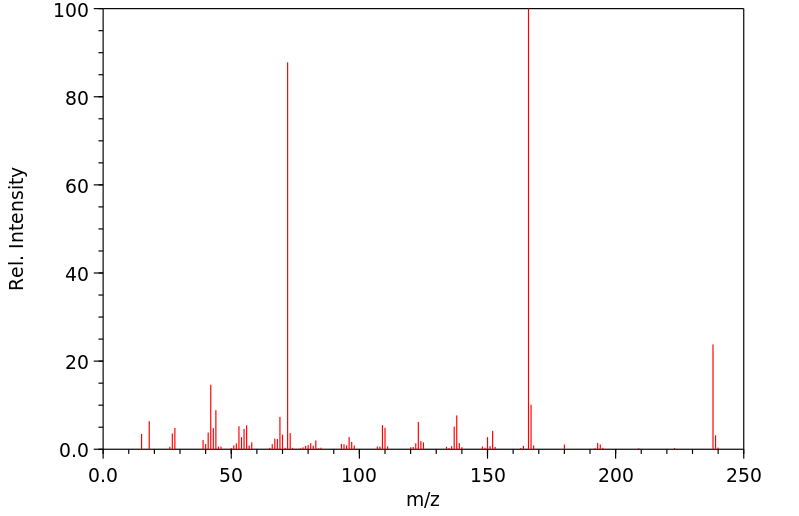
质谱MS
-
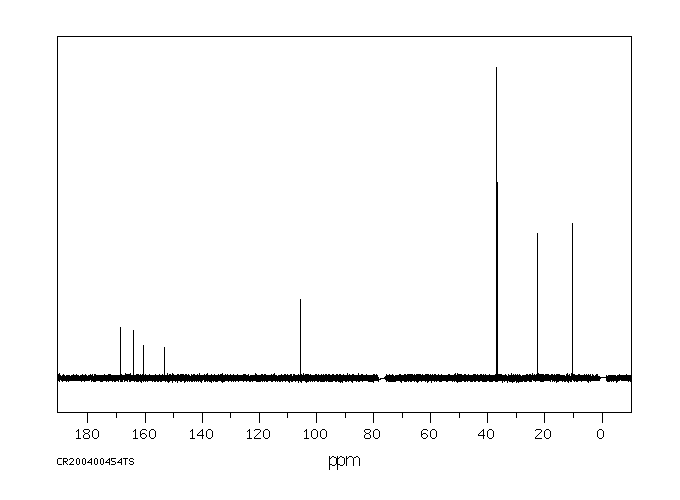
碳谱13CNMR
-
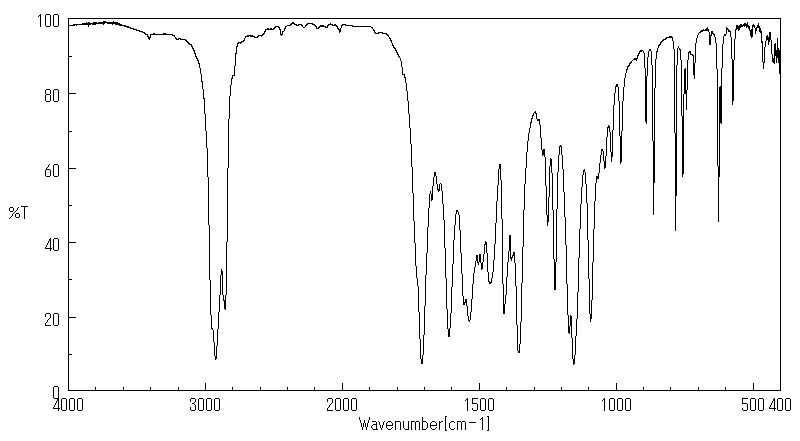
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息