1-(3-氯苯基)-3-苯基硫脲 | 4251-08-5
中文名称
1-(3-氯苯基)-3-苯基硫脲
中文别名
——
英文名称
1-phenyl-3-m-chlorophenyl thiocarbamide
英文别名
1-phenyl 3-m-chlorophenylthiocarbamide;1-(3-chlorophenyl)-3-phenylthiourea;N-m-chlorophenyl-N'-phenylthiourea;1-(3-chloro-phenyl)-3-phenyl-thiourea;N-(3-chloro-phenyl)-N'-phenyl-thiourea;N-(3-Chlor-phenyl)-N'-phenyl-thioharnstoff
CAS
4251-08-5
化学式
C13H11ClN2S
mdl
——
分子量
262.763
InChiKey
YRBVLVKDMVTDDO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120 °C
-
沸点:374.1±44.0 °C(Predicted)
-
密度:1.385±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:56.2
-
氢给体数:2
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N'-二苯基硫脲 N,N-diphenylthiourea 102-08-9 C13H12N2S 228.318 1-(3-氯苯基)-3-苯基脲 1-(3-chlorophenyl)-3-phenylurea 2008-71-1 C13H11ClN2O 246.696
反应信息
-
作为反应物:描述:参考文献:名称:Abuzar, Syed; Sharma, Satyavan; Iyer, R. N., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1980, vol. 19, # 3, p. 211 - 212摘要:DOI:
-
作为产物:描述:参考文献:名称:无催化剂和无溶剂条件下基于异氰化物的多组分反应,用于合成不对称硫脲摘要:通过芳族异氰酸酯,胺与1,2-二叔丁基二硫醚(DTBS)的顺序一锅三组分反应,开发了一种新的高效合成硫脲衍生物的方法,并在其中合成了27个不同的实例好到极好的产量。DTBS被认为是不同时使用催化剂和溶剂的有效硫替代物。该协议不使用任何过渡金属催化剂或特殊的实验装置。DOI:10.1016/j.tetlet.2016.11.082
文献信息
-
Catalytic Asymmetric Conjugate Addition and Sulfenylation of Diarylthiazolidin-2,4-diones作者:Lihui Jiao、Liwei Bu、Xinyi Ye、Xiaowei Zhao、Zhiyong JiangDOI:10.1021/acs.joc.6b01637日期:2016.10.21This work reports the first application of diarylthiazolidin-2,4-diones as nucleophiles in asymmetric catalysis. By utilizing chiral amino acid-based (thio)urea–tertiary amines as the catalysts, we successively established asymmetric conjugate addition to nitroolefins and sulfenylation to N-(sulfanyl)-succinimides of diarylthiazolidin-2,4-diones. Two series of biologically important 5-aryl-5-substituted
-
Electrocatalytic Desulfurizative Amination of Thioureas to Guanidines作者:Wei Jiang、Bing Wang、Chunlan Song、Jie LiuDOI:10.1021/acs.joc.3c01612日期:2023.10.20N-containing molecules with a wide range of applications. Described here is a selective and efficient electrochemical approach to the synthesis of guanidines from easily accessible thioureas and amines. The key to success for this reaction is the in situ generation of a hypervalent iodine reagent as a catalyst from iodoarene by anodic oxidation. This mild desulfurizative amination presents ample substrate scope
-
Uberhande; Thakare; Berad, Journal of the Indian Chemical Society, 2010, vol. 87, # 9, p. 1137 - 1141作者:Uberhande、Thakare、BeradDOI:——日期:——
-
Pandey,H.N.; Ram,V.J., Journal of the Indian Chemical Society, 1972, vol. 49, p. 171 - 173作者:Pandey,H.N.、Ram,V.J.DOI:——日期:——
-
Deshmukh; Agrawal; Deshmukh, Journal of the Indian Chemical Society, 2011, vol. 88, # 11, p. 1759 - 1762作者:Deshmukh、Agrawal、DeshmukhDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
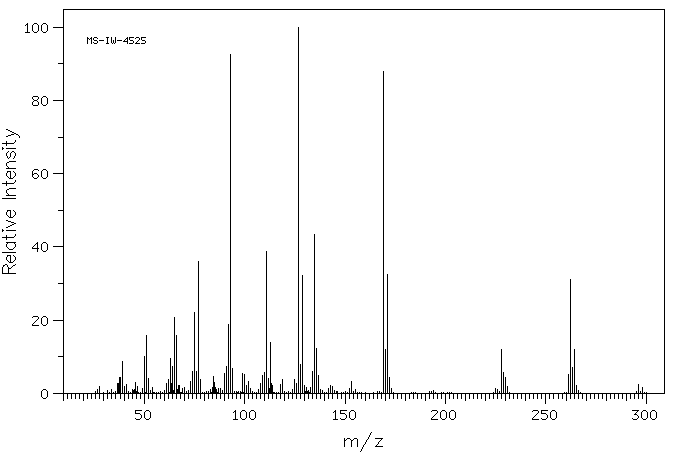
质谱MS
-
碳谱13CNMR
-
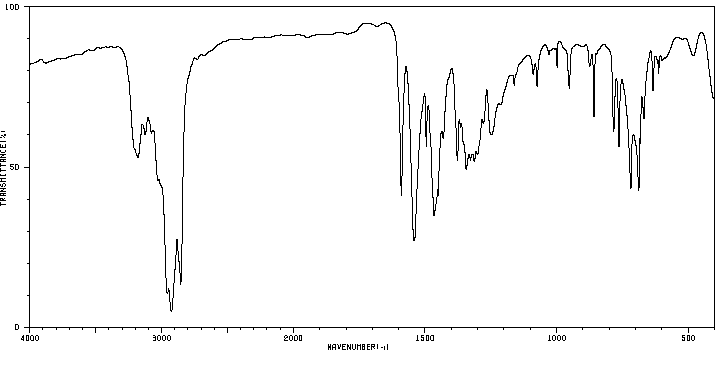
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫