2,5-二硫代联二脲 | 142-46-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:212 °C (dec.)(lit.)
-
沸点:291.6±23.0 °C(Predicted)
-
密度:1.412 (estimate)
-
物理描述:2,5-dithiobiurea is a brown powder. (NTP, 1992)
-
溶解度:less than 0.1 mg/mL at 73° F (NTP, 1992)
-
稳定性/保质期:
常温常压下稳定。禁配物:强氧化剂
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:140
-
氢给体数:4
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:OTH
-
RTECS号:EC1460000
-
海关编码:2930909090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封于干燥阴凉处保存。
SDS
| Name: | 2 5-Dithiobiurea 99% Material Safety Data Sheet |
| Synonym: | Bisthiocarbamyl Hydrazine; N,N'-Bisthiocarbamyl Hydrazine |
| CAS: | 142-46-1 |
Synonym:Bisthiocarbamyl Hydrazine; N,N'-Bisthiocarbamyl Hydrazine
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 142-46-1 | 2,5-Dithiobiurea | 99 | 205-537-6 |
Risk Phrases: 23/24/25 43 45
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. May cause sensitization by skin contact. May cause cancer.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May cause skin sensitization, an allergic reaction, which becomes evident upon re-exposure to this material.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Repeated or prolonged exposure may cause allergic reactions in sensitive individuals.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 142-46-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 200.00 - 203.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: negligible
Specific Gravity/Density:
Molecular Formula: C2H6N4S2
Molecular Weight: 150.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen sulfide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 142-46-1: EC1460000 LD50/LC50:
Not available.
Carcinogenicity:
2,5-Dithiobiurea - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 45 May cause cancer.
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 43 May cause sensitization by skin contact.
Safety Phrases:
S 53 Avoid exposure - obtain special instructions
before use.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 142-46-1: No information available.
Canada
CAS# 142-46-1 is listed on Canada's NDSL List.
CAS# 142-46-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 142-46-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
双硫脲纯品为白色结晶固体,熔点为206~208℃。它在热水中有一定的溶解度,并且易溶于吡啶、三乙胺、二甲基甲酰胺及一般溶剂中。
用途
双硫脲是杀菌剂叶枯唑的中间体,同时也是农药和医药中间体,并用于制作医药中间体。
生产方法
硫酸肼与硫氰酸胺经缩合、重排而得。其制备过程是在反应釜中于搅拌下投入湿基硫酸肼,加入少量催化剂,再投入硫氰酸铵,开启夹套蒸汽加热回流3.5小时后冷却,放料,过滤并用水洗涤,真空吸滤至干燥,最终得到成品。
类别
农药
毒性分级
高毒
急性毒性
口服-大鼠 LD50: 500 毫克/公斤;腹腔-小鼠 LD50: 100 毫克/公斤
可燃性危险特性
受热分解会排放有毒的氮氧化物和硫氧化物。
储运特性
应存放于通风低温干燥的库房,并与氧化剂、酸类分开。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氨基硫脲 thiosemicarbazide 79-19-6 CH5N3S 91.1368
反应信息
-
作为反应物:描述:参考文献:名称:2,5-二氨基-噻二唑/ N,N'-硫代碳酰肼的新型双衍生物的简便合成及其生物学前景摘要:通过绿色方法(声化学和微波)合成了一系列新的2,5-噻二唑和N,N'-硫代碳酰肼的新双衍生物及其过渡金属配合物,即,能源和时间效率高,操作比现有常规方法更简单。所有合成的化合物均通过常规分析技术充分表征,并进行了初步的生物学筛选。对这些新颖的表征化合物进行了抗菌活性筛选,并显示出令人鼓舞的结果。还测试了一些有效的化合物对MDA-MB231(人乳腺癌细胞系)的抗肿瘤活性,化合物5以潜在的抗肿瘤化合物的形式出现,其次是化合物2、10和9,IC 50值分别为29.48、32.25、32.34和34.34μg/ ml。为了了解这些化合物的结合相互作用,使用AUTODOCK 4.2套件进行了计算机研究,发现该结果支持实验结果。DOI:10.1007/s11164-018-3554-2
-
作为产物:描述:参考文献:名称:Synthesis and antimicrobical evaluation of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazolo[1,5-a]pyrimidine moiety摘要:A series of novel 1,3,4-thiadiazole derivatives bearing 1,2,4-triazolo[1,5-a]pyrimidine moiety were synthesized by the method of splicing active substructures. Among these derivatives, compounds 12,13,15-22 and 24-31 were firstly reported. All the compounds were assayed for antimicrobial activities against five fungi strains and four bacteria strains. The preliminary results indicated that compounds 25 and 28-31 showed good antifungal activities against Physaclospora piricola and Rhizoctonia solani. Compound 26 exhibited good antifungal activities against Cercospora beticola and R. solani. Most of the compounds showed better antibacterial activities against Gram-negative bacteria strains than Gram-positive bacteria strains. Compounds 25 and 28 showed the best activities against Pseudomonas fluorescence while compounds 30-31 showed good activities against Escherichia coli. (C) 2013 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2013.04.014
文献信息
-
Sulphonyl-1,2,4-triazole salts申请人:Phostech Lithium Inc.公开号:US07919629B2公开(公告)日:2011-04-05The invention relates to triazole salts, to their preparation and to applications thereof. The salts have at least one anionic triazolium group which carries at least one chlorosulphonyl, fluorosulphonyl or alkoxyfluorosulphonyl group, each of the anionic groups being combined with a proton or a cation that has a valency of less than or equal to 4. The salts are useful as synthesis reagents, as chemical-reaction or polymerization catalysts, and as ion-conducting materials for electrochemical generators, supercapacitors and electrochromic devices.
-
A Model for Using Novel Nursing Interventions to Meet the Challenges of Community Health Needs作者:Julie A. Koehler、Jennifer Cha、Carol E. SmithDOI:10.1177/1084822302014002007日期:2002.2
The aging population growth, shortened hospital stays, reduction in home care services reimbursement, and the looming nursing shortage will all drastically increase community health care needs and caregiving challenges. Unique and traditional methods of care delivery provided via innovative interventions can be used to address these needs. Many research-based nursing interventions can be administered at a distance (e.g., video, telehealth, and even robotic “nurses”) or without the nurse being present through volunteers or delegation, thus increasing communitybased care. A model categorizing and guiding use of these nursing interventions is described herein.
人口老龄化增长、住院时间缩短、家庭护理服务费用减少以及迫在眉睫的护理人员短缺将大幅增加社区医疗需求和护理挑战。通过创新干预提供独特和传统的护理方法可以解决这些需求。许多基于研究的护理干预可以远程实施(例如视频、远程医疗,甚至是机器人“护士”),或者在护士不在场的情况下通过志愿者或委派进行,从而增加社区护理。本文描述了一个对这些护理干预进行分类和指导使用的模型。 -
Heterocyclic compounds from urea derivatives. Part XVIII. Adducts from aminoguanidines and aroyl isothiocyanates and their cyclisation作者:Frederick KurzerDOI:10.1039/j39700001805日期:——1-Amidino-4-benzoyl(thiosemicarbazide) hydrochloride is cyclised by hydrochloric or orthophosphoric acid to 2-amino-5-benzamido-1,3,4-thiadiazole, by acetic anhydride to the corresponding acetyl derivative, and by alkali to 3-mercapto-5-phenyl-1,2,4-triazole. 1-Amino-2-phenylguanidine salts undergo an analogous series of reactions. The mechanism of these addition–cyclisations is discussed and correlated
-
Reactions of thioamides and alkali metal salts of dithiocarbamates with 2-bromo-7-methyl-5-oxo-5H-1,3,4-thiazolo[3,2-a]pyrimidine and antimicrobial activity of products作者:M. A. Kukaniev、T. M. Salimov、M. S. Murvatulloeva、I. Kh. ImatshoevDOI:10.1007/s11094-006-0144-1日期:2006.8A series of thiuronium salts have been synthesized via reactions of various thioamides with 2-bromo-7-methyl-5-oxo-5H-1,3,4-thiazolo[3,2-a]pyrimidine. The products have been characterized with respect to antimicrobial activity.
-
Synthesis and properties of azothiazole based π-conjugated polymers作者:Zhuangqing Yan、Bin Sun、Chang Guo、Yuning LiDOI:10.1039/c4tc00809j日期:——
Azothiazole (ATz) is used for the first time as an electron-acceptor building block for donor–acceptor polymers used in OTFTs.
Azothiazole (ATz)首次被用作电子受体建筑模块,用于用于OTFTs的给体-受体聚合物中。
表征谱图
-
氢谱1HNMR
-
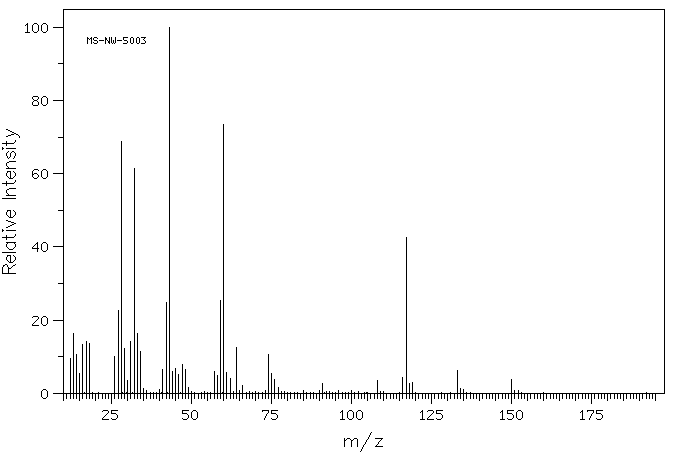
质谱MS
-
碳谱13CNMR
-
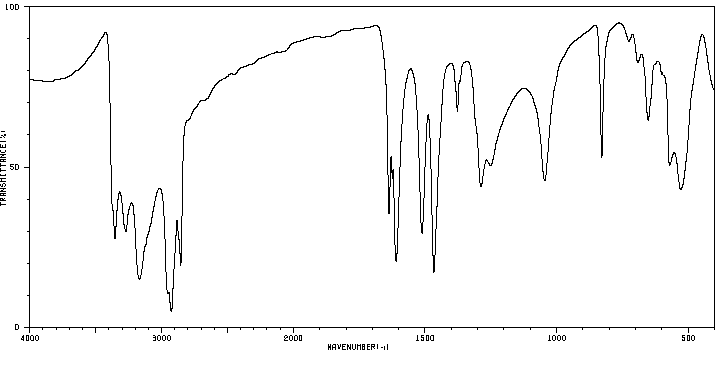
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息