溴化3-烯丙基苯并噻唑翁 | 16407-55-9
中文名称
溴化3-烯丙基苯并噻唑翁
中文别名
溴化3-烯丙基苯并噻唑鎓;N-烯丙基苯并噻唑鎓溴化物
英文名称
N-allylbenzothiazolium bromide
英文别名
3-prop-2-enyl-1,3-benzothiazol-3-ium;bromide
CAS
16407-55-9
化学式
Br*C10H10NS
mdl
——
分子量
256.166
InChiKey
MEYXQRPXVJWXBZ-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:150 °C
计算性质
-
辛醇/水分配系数(LogP):-0.62
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:32.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
储存条件:室温且干燥
SDS
N-烯丙基苯并噻唑鎓溴化物
模块 1. 化学品
产品名称: N-Allylbenzothiazolium Bromide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-烯丙基苯并噻唑鎓溴化物
百分比: >98.0%(T)
CAS编码: 16407-55-9
分子式: C10H10BrNS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-烯丙基苯并噻唑鎓溴化物
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
150°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 甲醇
N-烯丙基苯并噻唑鎓溴化物
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-烯丙基苯并噻唑鎓溴化物
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: N-Allylbenzothiazolium Bromide
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-烯丙基苯并噻唑鎓溴化物
百分比: >98.0%(T)
CAS编码: 16407-55-9
分子式: C10H10BrNS
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-烯丙基苯并噻唑鎓溴化物
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
150°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 甲醇
N-烯丙基苯并噻唑鎓溴化物
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-烯丙基苯并噻唑鎓溴化物
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:溴化3-烯丙基苯并噻唑翁 在 1,8-二氮杂双环[5.4.0]十一碳-7-烯 作用下, 以 甲醇 为溶剂, 反应 48.0h, 生成 1-(1,3-Benzothiazol-2-yl)-1-(2,4-dimethylphenyl)but-3-en-1-ol参考文献:名称:通过克莱森重排拦截布雷斯洛中间体:无需有机金属试剂合成复杂的叔醇摘要:报道了一种新的克莱森重排,其中 Breslow 中间体作为羟基取代的N,S-烯酮缩醛参与,以提供复杂的 3° 醇,而无需使用有机金属试剂。该反应构成了 Breslow 中间体前所未有的反应模式。DOI:10.1021/ol303053c
-
作为产物:参考文献:名称:一步步进入钌与混合配体的烯烃链状N,S-杂环卡宾配合物†摘要:一系列Ru(II)混合配体络合物RuBr 2(PPh 3)2(N -AyBzTh)(Ay =丙-2-烯; BzTh =苯并噻唑-2-亚烷基)(4),RuBr(OAc)(PPh 3)(N -MeAyBzTh)(5),RuBr(OAc)(PPh 3)(N -MeBnBzTh)2(MeBn = 3-甲基丁-2-烯基)(6)和RuCl(OAc)(PPh 3)(N -MeBnBzTh)(7)由Ru(OAc)2(PPh 3)2合成一锅缩合和配体交换反应。透视单晶衍射分析表明,它们是中性八面体的Ru(II)配合物与一个或两个Ñ,š -杂环卡宾(NSHC)配体和一个协调(4,5和7)或悬挂(6)烯烃臂。系统显示一系列自选结构变化。和混合烯烃NSHC的条目卤化物配体弹出操作任一个(5,6和7)的膦配体或两者保持膦(4)代替醋酸盐。通过保持烯烃侧基(6),也可以容纳两个NSHC配体。配合物5和7是同构的,在配DOI:10.1039/c2dt12354a
-
作为试剂:描述:3-吡啶甲醛 、 天然维生素E 在 叔丁基过氧化氢 、 potassium tert-butylate 、 溴化3-烯丙基苯并噻唑翁 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 36.0h, 以85%的产率得到VE烟酸酯参考文献:名称:一种生育酚烟酸酯的合成方法摘要:本发明属于有机化学合成技术领域,具体涉及一种由3‑吡啶甲醛与生育酚制备生育酚烟酸酯的方法。本发明本发明以3‑吡啶甲醛与生育酚为原料,氮杂卡宾为催化剂,通过氧化酯化合成生育酚烟酸酯。本发明原料廉价、反应条件温和、工艺简便,绿色安全,高效环保,适用于工业化生产。公开号:CN113563317A
文献信息
-
Stoichiometric sensitivity and structural diversity in click-active copper(<scp>i</scp>) N,S-heterocyclic carbene complexes作者:Xiaoyan Han、Zhiqiang Weng、David James Young、Guo-Xin Jin、T. S. Andy HorDOI:10.1039/c3dt52059e日期:——A series of novel Cu(I) N,S-heterocyclic carbene (NSHC) complexes [Cu(μ-Br)(NSHC)]2, [Cu(μ-X)(NSHC)]4 (X = Br or I), [(NSHC)2Cu(μ-Br)2Cu(NSHC)], and [(NSHC)2CuBr] have been isolated from in situ generated CuOtBu and N-substituted benzothiazolium halides and characterized by X-ray crystallography. Five structural motifs were observed, viz. MxLy where x : y = 2 : 2, 4 : 4, 2 : 3, 1 : 2 and 2 : 4, with一系列新颖的Cu(I)N,S-杂环卡宾(NSHC)配合物[Cu(μ-Br)(NSHC)] 2,[Cu(μ-X)(NSHC)] 4(X = Br或I) ,[(NSHC)2 Cu(μ-Br)2 Cu(NSHC)]和[(NSHC)2 CuBr]已从原位生成的CuO t Bu和N-取代的苯并噻唑鎓卤化物中分离出来,并通过X射线晶体学表征。观察到五个结构基序,即。M x L y其中x : y= 2:2、4:4、2:3、1:2和2:4,Cu⋯Cu的间距跨度范围为2.5626(7)至3.4725(7)Å。对这些化合物的催化活性的初步研究表明,不寻常的单核络合物6 [(NSHC)2 CuBr]是叠氮化物和炔烃的Huisgen 1,3-偶极环加成反应的活性催化剂,而络合物1-5和7则微不足道。不太活跃。
-
Radical [1,3] Rearrangements of Breslow Intermediates作者:Sefat Alwarsh、Yi Xu、Steven Y. Qian、Matthias C. McIntoshDOI:10.1002/anie.201508368日期:2016.1.4bear radical‐stabilizing N substituents, such as benzyl, cinnamyl, and diarylmethyl, undergo facile homolytic CN bond scission under mild conditions to give products of formal [1,3] rearrangement rather than benzoin condensation. EPR experiments and computational analysis support a radical‐based mechanism. Implications for thiamine‐based enzymes are discussed.
-
Pd(ii) complexes of N,S-heterocyclic carbenes with pendant and coordinated allyl function and their Suzuki coupling activities作者:Swee Kuan Yen、Lip Lin Koh、Han Vinh Huynh、T. S. Andy HorDOI:10.1039/b706968e日期:——3-(2-Propenyl)benzothiazolium bromide (A) provides a direct and simple entry to Pd(II) complexes with N,S-heterocyclic carbene (NSHC) ligands functionalized with an allyl pendant with hemilabile potential. Addition of salt A to Pd(OAc)2 eliminates HOAc and affords the bis(carbene) complexes cis-[PdBr2(NHSC)2] (cis-1, NSHC = 3-(2-propenyl)benzothiazolin-2-ylidene) and trans-[PdBr2(NHSC)2] (trans-1) along with the monocarbene complexes [PdBr2(NSHC)] (2) and trans-[PdBr2(benzothiazole-κN)(NSHC)] (3) as minor side products. Salt-metathesis of cis-1 with AgO2CCF3 yields the mixed dicarboxylato-bis(carbene) complex cis-[Pd(O2CCF3)2(NSHC)2] (4). Complexes cis-1, trans-1 and 4 were characterized by multinuclear NMR spectroscopies, ESI mass spectrometry and elemental analysis. The molecular structures of complexes cis-1, 2 and 3 have been determined by X-ray single crystal diffraction. Complexes cis-1 and 4 as well as an in situ mixture of Pd(OAc)2 and salt A are active toward Suzuki–Miyaura coupling of aryl bromides and activated aryl chlorides giving good conversions.溴化3-(2-丙烯基)苯并噻唑鎓盐(A)为制备含有功能化的烯丙基半可键合N,S-杂环卡宾(NSHC)配体的钯(II)配合物提供了一个直接且简单的途径。将盐A加入到Pd(OAc)2中会消除HOAc,并得到双卡宾配合物cis-[PdBr2(NHSC)2](cis-1,其中NSHC=3-(2-丙烯基)苯并噻唑啉-2-亚基)和trans-[PdBr2(NHSC)2](trans-1),同时还产生次要副产物单卡宾配合物[PdBr2(NSHC)](2)和trans-[PdBr2(苯并噻唑-κN)(NSHC)](3)。通过与AgO2CCF3进行盐交换反应,cis-1生成了混合二羧酸根-双卡宾配合物cis-[Pd(O2CCF3)2(NSHC)2](4)。配合物cis-1、trans-1和4通过多核NMR光谱学、ESI质谱法和元素分析进行了表征。配合物cis-1、2和3的分子结构通过X射线单晶衍射确定。配合物cis-1和4以及Pd(OAc)2和盐A的原位混合物对于Suzuki–Miyaura交叉偶联反应中的芳基溴和活化芳基氯具有活性,提供了良好的转化率。
-
Silver halide emulsion containing an alkenyl benzothiazolium salt as申请人:Fuji Photo Film Co., Ltd.公开号:US03954478A1公开(公告)日:1976-05-04A silver halide light-sensitive material which comprises a light-sensitive silver halide emulsion containing a compound represented by the following formula (I): ##SPC1## wherein R.sub.1, R.sub.2, R.sub.3, R.sub.4 and R.sub.5 each represents a hydrogen atom or a lower alkyl group; and R.sub.6 and R.sub.7 each represents a hydrogen atom, a lower alkyl group or a lower alkoxy group or can combine to form a condensed ring, and X.sup.- represents an anion.
-
Silver halide multilayer color photographic element comprising a申请人:Minnesota Mining and Manufacturing Company公开号:US05212056A1公开(公告)日:1993-05-18A multilayer silver halide color photographic element comprising at least one blue-sensitive silver halide emulsion layer comprising a blue spectral sensitizing dye and a supersensitizing amount of a disulfide compound of formula ##STR1## wherein R.sub.1, R.sub.2, R.sub.3 and R.sub.4, equal or different, each represents a hydrogen atom or a lower alkyl group, R.sub.5 represents a hydrogen atom, a formyl group or an acetyl group, R.sub.6 and R.sub.7, equal or different, each represents a hydrogen atom or a lower alkyl group, or R.sub.6 and R.sub.7 represent the elements needed to complete an unsaturated cyclic nucleus. The combination provides a blue-sensitive silver halide emulsion layer having high sensitivity, low fog and good resistance against fading of the latent image.
表征谱图
-
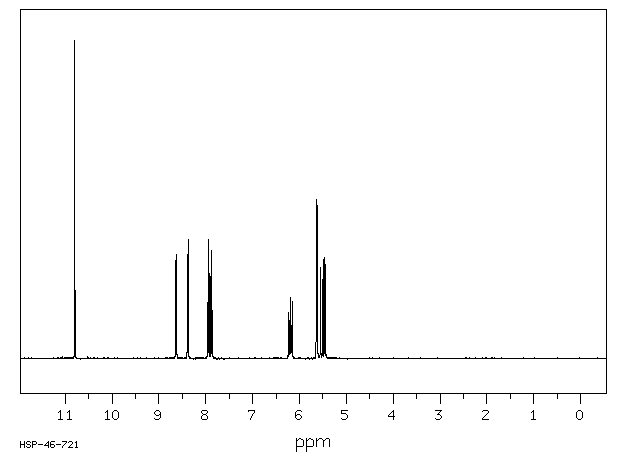
氢谱1HNMR
-
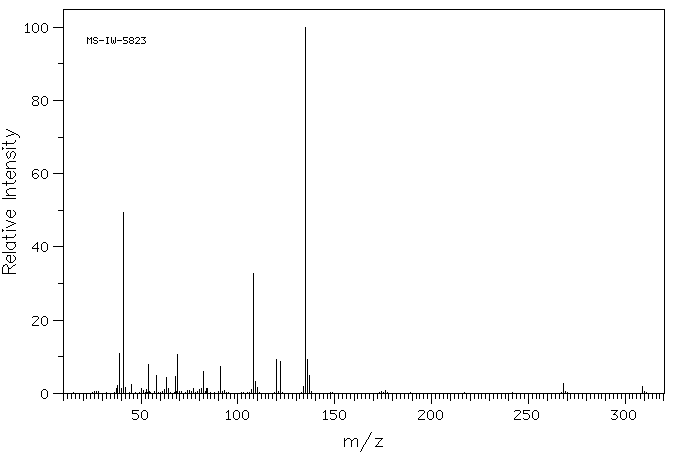
质谱MS
-
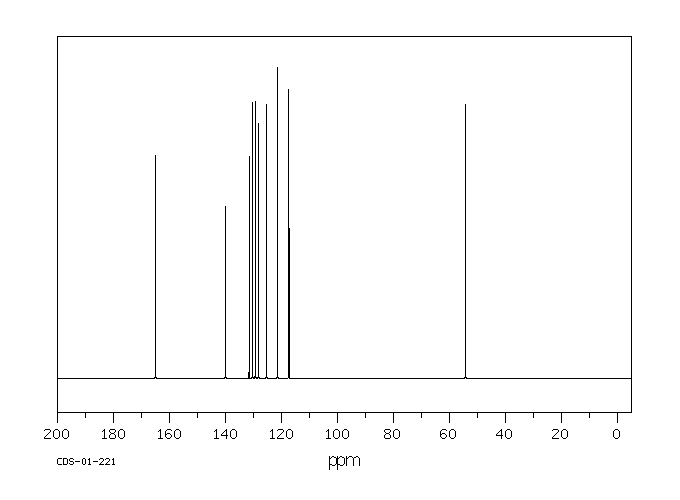
碳谱13CNMR
-
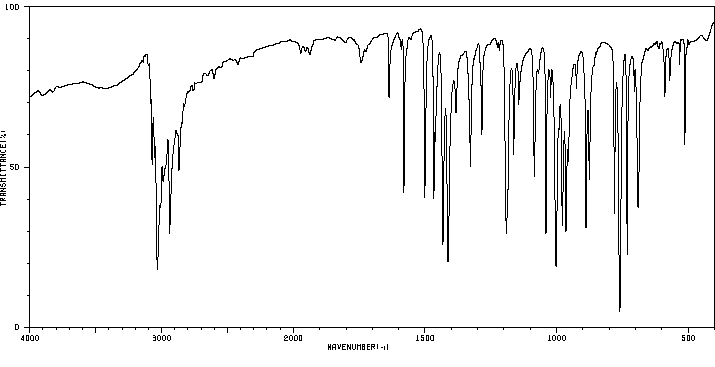
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z)-1-(3-乙基-5-羟基-2(3H)-苯并噻唑基)-2-丙酮
齐拉西酮砜
齐帕西酮-d8
阳离子蓝NBLH
阳离子荧光黄4GL
锂2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
铜酸盐(4-),[2-[2-[[2-[3-[[4-氯-6-[乙基[4-[[2-(硫代氧代)乙基]磺酰]苯基]氨基]-1,3,5-三嗪-2-基]氨基]-2-(羟基-kO)-5-硫代苯基]二氮烯基-kN2]苯基甲基]二氮烯基-kN1]-4-硫代苯酸根(6-)-kO]-,(1:4)氢,(SP-4-3)-
铜羟基氟化物
钾2-(4-氨基苯基)-5-甲基-1,3-苯并噻唑-7-磺酸酯
钠3-(2-{(Z)-[3-(3-磺酸丙基)-1,3-苯并噻唑-2(3H)-亚基]甲基}[1]苯并噻吩并[2,3-d][1,3]噻唑-3-鎓-3-基)-1-丙烷磺酸酯
邻氯苯骈噻唑酮
西贝奈迪
螺[3H-1,3-苯并噻唑-2,1'-环戊烷]
螺[3H-1,3-苯并噻唑-2,1'-环己烷]
葡萄属英A
草酸;N-[1-[4-(2-苯基乙基)哌嗪-1-基]丙-2-基]-2-丙-2-基氧基-1,3-苯并噻唑-6-胺
苯酰胺,N-2-苯并噻唑基-4-(苯基甲氧基)-
苯酚,3-[[2-(三苯代甲基)-2H-四唑-5-基]甲基]-
苯胺,N-(3-苯基-2(3H)-苯并噻唑亚基)-
苯碳杂氧杂脒,N-1,2-苯并异噻唑-3-基-
苯甲酸,4-(6-辛基-2-苯并噻唑基)-
苯甲基2-甲基哌啶-1,2-二羧酸酯
苯并噻唑正离子,2-[3-(1,3-二氢-1,3,3-三甲基-2H-吲哚-2-亚基)-1-丙烯-1-基]-3-乙基-,碘化(1:1)
苯并噻唑正离子,2-[2-[4-(二甲氨基)苯基]乙烯基]-3-乙基-6-甲基-,碘化
苯并噻唑正离子,2-[(2-乙氧基-2-羰基乙基)硫代]-3-甲基-,溴化
苯并噻唑啉
苯并噻唑三氯金(III)
苯并噻唑-d4
苯并噻唑-7-乙酸
苯并噻唑-6-腈
苯并噻唑-5-羧酸
苯并噻唑-5-硼酸频哪醇酯
苯并噻唑-4-醛
苯并噻唑-4-乙酸
苯并噻唑-2-磺酸钠
苯并噻唑-2-磺酸
苯并噻唑-2-磺酰氟
苯并噻唑-2-甲醛
苯并噻唑-2-甲酸
苯并噻唑-2-甲基甲胺
苯并噻唑-2-基磺酰氯
苯并噻唑-2-基甲基-乙基-胺
苯并噻唑-2-基叠氮化物
苯并噻唑-2-基-邻甲苯-胺
苯并噻唑-2-基-己基-胺
苯并噻唑-2-基-(4-氯-苯基)-胺
苯并噻唑-2-基-(4-氟-苯基)-胺
苯并噻唑-2-基-(4-乙氧基-苯基)-胺
苯并噻唑-2-基-(2-甲氧基-苯基)-胺
苯并噻唑-2-基-(2,6-二甲基-苯基)-胺