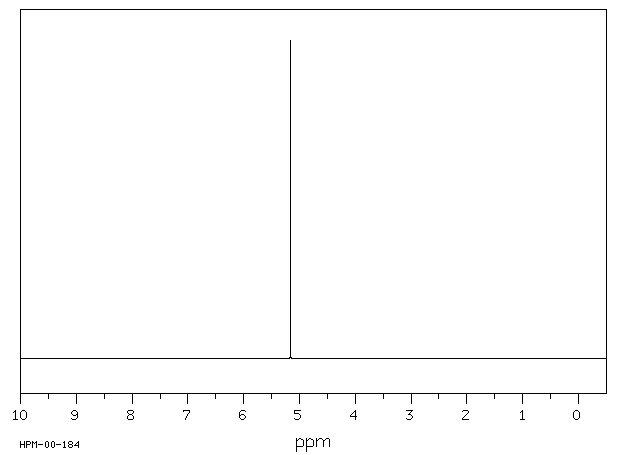
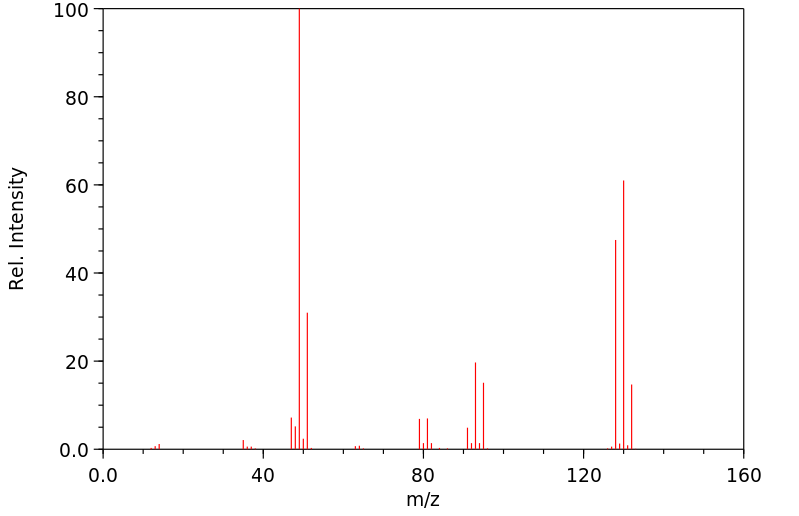
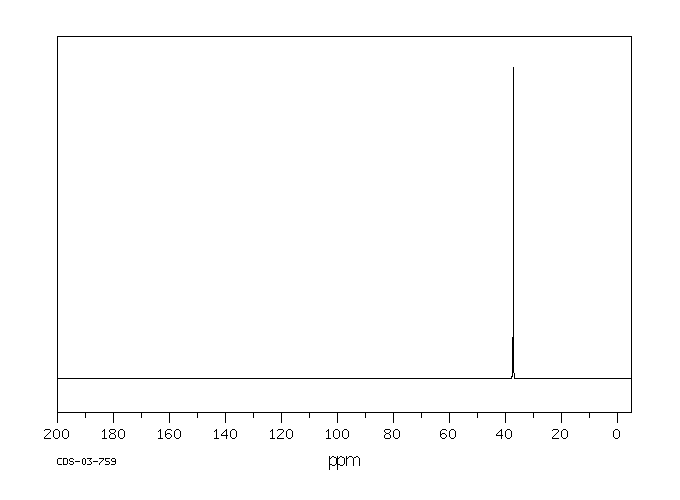
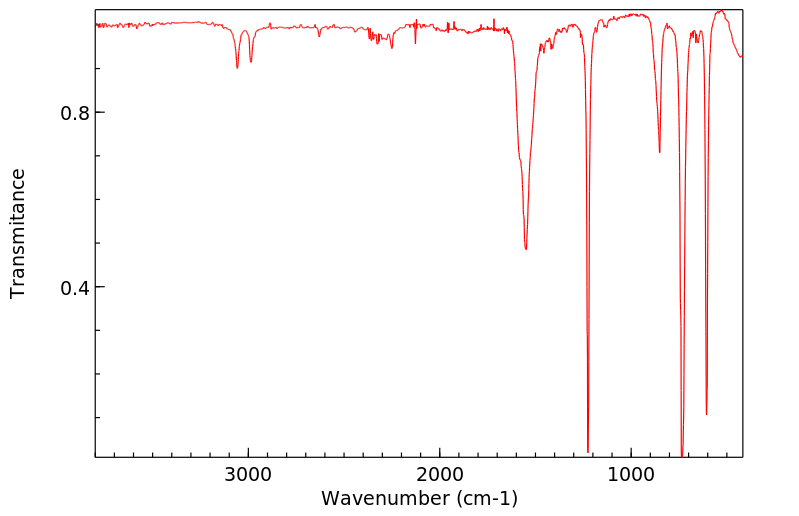
代谢
血液中的无机溴化物和尿液中的无机溴化物是在暴露于空气中1000 ppm氯甲溴的狗身上确定的。这些动物每天暴露7小时,每周5天。在第三周,血清中的无机溴化物从正常的5至10毫克/100毫升增加到超过200毫克。在第13周和第14周,浓度超过了每100毫升血液300毫克的无机溴化物。... 挥发性溴化物的浓度被确定,以每100毫升血液中毫克氯甲溴的形式表示。在暴露结束时立即采集,观察到每100毫升血液中有5至9毫克的氯甲溴浓度。在最后一次暴露结束后的17-65小时内,一只狗中没有观察到挥发性溴化物,另一只狗中的浓度小于1毫克。似乎氯甲溴在空气中暴露于蒸汽时出现在血液中,但在暴露停止后会迅速消失。显然,大量的物质被水解或代谢生成无机溴化物...。
... Inorganic bromide in blood serum and urine /was determined/ in dogs exposed to 1000 ppm of methylene chlorobromide in air. These animals were exposed 7 hr/day, 5 days/week. During the third week, the serum inorganic bromide had increased from a normal of 5 to 10 mg/100 mL to >200 mg. By the 13th and 14th weeks, the concentration was greater than 300 mg of inorganic bromide per 100 mL of blood. ... The ... concentration of volatile bromide /was determined/ expressed as milligrams of methylene chlorobromide per 100 mL of blood. Taken immediately at the end of the exposure, concentrations between 5 and 9 mg of methylene chlorobromide per 100 mL were observed. At periods of 17-65 hr after the end of the last exposure, no volatile bromide was observed in one dog and concentrations of <1 mg in the other. It would appear that methylene chlorobromide as such appears in the blood during exposure to vapors in air, but disappears rapidly on cessation of exposure. Apparently, a significant amount of material is hydrolyzed or metabolized to yield inorganic bromide ... .
来源:Hazardous Substances Data Bank (HSDB)