S-ethyl O-methyl carbonate | 38103-96-7
中文名称
——
中文别名
——
英文名称
S-ethyl O-methyl carbonate
英文别名
O-Methyl S-Ethyl Thiocarbonate;S-ethylsulfenyl O-methyl thiocarbonate;O-Methyl-S-ethylthiolcarbonat;S-Aethyl-methyl-monothiocarbonat;S-Ethyl o-methyl thiocarbonate;methyl ethylsulfanylformate
CAS
38103-96-7
化学式
C4H8O2S
mdl
——
分子量
120.172
InChiKey
VVIAWLIDZAXESO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:142.6±23.0 °C(Predicted)
-
密度:1.072±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:51.6
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:参考文献:名称:消除热量的机制。第15部分。由于羰基的亲核性增强,碳酸烷基甲基碳酸酯和S-烷基O-甲基碳酸酯的热解速率分布异常摘要:对于每种化合物,已在50 K的范围内测量了热解消除碳酸乙酯,异丙酯和叔丁基碳酸酯以及碳酸二叔丁酯的速率系数。600 K下的相对速率为1:29.6:2 934:3 526,伯,仲和叔化合物的扩散速率与消除一系列其他酯(包括碳酸烷基苯酯)所得的速率不一致。发现反应性最低的化合物比预期的反应性更高,这可能是由于其过渡态的较高E i特性和碳酸二烷基酯中羰基的高亲核性共同造成的。S-乙基,S-异丙基和S-叔丁基O的热解速率数据-甲基碳酸盐给予在600 k中的相对速率为1:22:1 074的卜吨:PR我速率比(49:1),因此比镨更大我:Et比率,与所有其他相关抵销一样;这证实了文献结果(显示出相反的结果)是错误的。与羰基亲核性的重要性相比,硫醇乙酸酯和硫代碳酸酯与它们的含氧类似物相比看似异常的相对反应性也与酯热解中过渡态的可变极性的影响相一致,这也解释了硫醇,硫代和二硫代乙酸酯的相对反应性。对于碳酸DOI:10.1039/p29830000291
-
作为产物:参考文献:名称:消除热量的机制。第15部分。由于羰基的亲核性增强,碳酸烷基甲基碳酸酯和S-烷基O-甲基碳酸酯的热解速率分布异常摘要:对于每种化合物,已在50 K的范围内测量了热解消除碳酸乙酯,异丙酯和叔丁基碳酸酯以及碳酸二叔丁酯的速率系数。600 K下的相对速率为1:29.6:2 934:3 526,伯,仲和叔化合物的扩散速率与消除一系列其他酯(包括碳酸烷基苯酯)所得的速率不一致。发现反应性最低的化合物比预期的反应性更高,这可能是由于其过渡态的较高E i特性和碳酸二烷基酯中羰基的高亲核性共同造成的。S-乙基,S-异丙基和S-叔丁基O的热解速率数据-甲基碳酸盐给予在600 k中的相对速率为1:22:1 074的卜吨:PR我速率比(49:1),因此比镨更大我:Et比率,与所有其他相关抵销一样;这证实了文献结果(显示出相反的结果)是错误的。与羰基亲核性的重要性相比,硫醇乙酸酯和硫代碳酸酯与它们的含氧类似物相比看似异常的相对反应性也与酯热解中过渡态的可变极性的影响相一致,这也解释了硫醇,硫代和二硫代乙酸酯的相对反应性。对于碳酸DOI:10.1039/p29830000291
文献信息
-
Chemoselective Oxidation of Sulfides to Sulfoxides Using<i>N</i>-<i>t</i>-Butyl-<i>N</i>-chlorocyanamide作者:Vinod Kumar、Mahabir Parshad KaushikDOI:10.1246/cl.2005.1230日期:2005.9A simple, efficient and highly chemoselective method has been developed for the synthesis of sulfoxides from sulfides using N-t-butyl-N-chlorocyanamide as an oxidant in a mixed acetonitrile–water solution with a 1:1 mol ratio of oxidant to sulfides. N-t-butyl-N-chlorocyanamide, a source of positive chlorine, has been used for conversion of the variety of sulfides into their corresponding sulfoxides in quantitative yields.
-
Carbonate pyrolysis. Part 5. The gas-phase pyrolysis of some unsymmetrical monothiolcarbonates and a rationalisation of the rates of some related reactions作者:Nouria Al-Awadi、David B. BigleyDOI:10.1039/p29790000497日期:——Thiolcarbonates pyrolyse more slowly than carbonates. Where the sulphur atom is attached to the group which will form the olefin, this difference amounts to three orders of magnitude; when the sulphur atom is in the other ether position it is only ten-fold. These rate changes are discussed in terms of similar substitutions in thioacetates.
-
A general strategy for elaboration of the dithiocarbonyl functionality, -(C:O)SS-: application to the synthesis of bis(chlorocarbonyl)disulfane and related derivatives of thiocarbonic acids作者:George Barany、Alayne L. Schroll、Andrew W. Mott、David A. HalsrudDOI:10.1021/jo00172a056日期:1983.12
-
BARANY, G.;SCHROLL, A. L.;MOTT, A. W.;HALSRUD, D. A., J. ORG. CHEM., 1983, 48, N 24, 4750-4761作者:BARANY, G.、SCHROLL, A. L.、MOTT, A. W.、HALSRUD, D. A.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
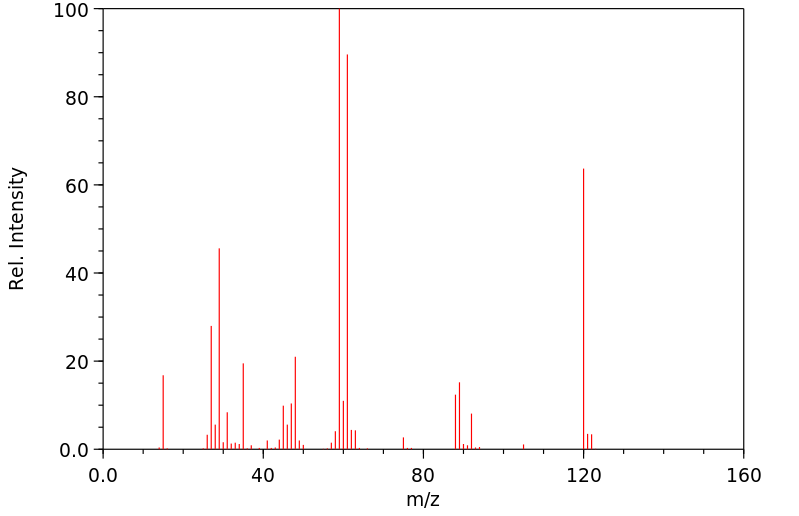
质谱MS
-
碳谱13CNMR
-
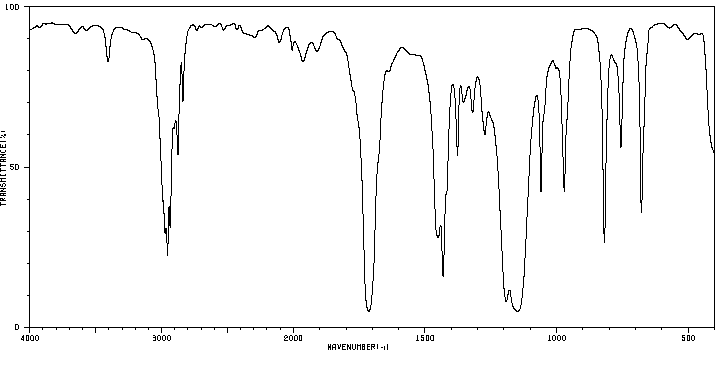
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
雷尼替丁EP杂质J
苯乙酮乙烷-1,2-二基二硫代缩醛
苯丙酮乙烷-1,2-二基二硫代缩醛
磷亚胺酸,[2,3,4,5,6-五氯-2,3,5,6-四氟-1-(2,2,3,3-四氟丙氧基)-4-(三氟甲基)环己基]-,三(2,2,3,3-四氟丙基)酯
硫代磷酸O,O-二乙基S-[2,2-二(乙硫基)丙基]酯
硫代二碳酸叔丁基乙基酯
硫代二碳酸 1-乙基 3-异丙基酯
甲硫基甲酸叔丁酯
甲氧基甲基硫烷基乙烷
甲氧基二硫代甲酸甲酯
甲氧基(甲基硫烷基)甲烷
甲基二[[(二甲基氨基)硫代甲酰]硫代]乙酸酯
甲基8-氧代-6,10-二硫杂螺[4.5]癸烷-7-羧酸酯
环辛酮硫代缩酮
环线威
环己基甲硫基甲基醚
环己基二乙酸二乙酯
氰硫基酸,2,2,2-三氯乙基酯
双(硫代甲氧基甲基)硫醚
双(亚甲基二硫代)四硫富瓦烯
六氢-2'3A-二甲基螺[1,3-二硫环戊并[4,5-B]呋喃-2,3'(2'H)-呋喃]
亚甲基二(氰基亚胺硫代碳酸甲酯)
亚甲基二(二异丁基二硫代氨基甲酸酯)
二邻茴香醚
二硫氰基甲烷
二硫代丁酸甲酯
二甲硫基甲烷
二甲氧基-[(2-甲基-1,3-氧硫杂环戊烷-2-基)甲硫基]-巯基膦烷
二异丙基黄原酸酯
二(硫代碳酸 O-丁基酯)硫代酸酐
二(二甲基二硫代氨基甲酸)亚甲基酯
二(乙硫基)甲烷
二(乙硫基)乙酸乙酯
二(乙氧基硫代羰基)硫醚
二(2-氨基乙基硫基)甲烷
乙醛,二(甲硫基)-
乙酸甲硫甲酯
乙氧基甲基异硫脲盐酸盐
乙丙二砜
乙丁二砜
丙烷-2、2-二基双(磺胺二基)二乙胺
丙烷-2,2-二基双(硫)基]二乙酸
三硫丙酮
[(异丙氧基硫基甲酰基硫基)硫基甲酰基硫基]硫代甲酸O-异丙基酯
[(N,N-二甲基二硫代氨基甲酰)甲基]甲基氰基亚氨二硫代碳酸酯
[(2R,4S,6R)-4,6-二甲基-1-硫羟基-1,3-二硫烷-2-基](二苯基)磷烷
[(2-羧基乙氧基)甲基]二甲基-锍溴化物(1:1)
S-甲基O-(2-甲基丙基)二硫代碳酸酯
S-烯丙基 O-戊基二硫代碳酸酯
S-乙基O-(1-碘乙基)硫代碳酸酯