N-benzyl-N'-phenyl-p-phenylenediamine | 13556-73-5
中文名称
——
中文别名
——
英文名称
N-benzyl-N'-phenyl-p-phenylenediamine
英文别名
N-Benzyl-N'-phenyl-p-phenylendiamin;N-phenyl-N'-benzyl-p-phenylenediamine;Phenyl-<4-benzylamino-phenyl>-amin;4-Benzylamino-diphenylamin;N-Benzyl-N'-phenyl-p-phenylenediamine;1-N-benzyl-4-N-phenylbenzene-1,4-diamine
CAS
13556-73-5
化学式
C19H18N2
mdl
——
分子量
274.365
InChiKey
CKVFMHUYNFWPAY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:21
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:24.1
-
氢给体数:2
-
氢受体数:2
反应信息
-
作为反应物:描述:4-(氯磺酰)苯甲酸 、 N-benzyl-N'-phenyl-p-phenylenediamine 在 三乙胺 作用下, 以 乙醇 为溶剂, 反应 18.0h, 生成 4-[(4-Anilinophenyl)-benzylsulfamoyl]benzoic acid参考文献:名称:Synthesis and pharmacological characterization of benzenesulfonamides as dual species inhibitors of human and murine mPGES-1摘要:The microsomal prostaglandin E-2 synthase 1 (mPGES-1) became a desirable target in recent years for the research of new anti-inflammatory drugs. Even though many potent inhibitors of human mPGES-1, tested in vitro assay systems, have been synthesized, they all failed in preclinical trials in rodent models of inflammation, due to the lack of activity on rodent enzyme. Within this work we want to present a new class of mPGES-1 inhibitors derived from a benzenesulfonamide scaffold with inhibitory potency on human and murine mPGES-1. Starting point with an IC50 of 13.8 mu M on human mPGES-1 was compound 1 (4-{benzyl[(4-methoxyphenyl) methyl] sulfamoyl} benzoic acid; FR4), which was discovered by a virtual screening approach. Optimization during a structure-activity relationship (SAR) process leads to compound 28 (4-[(cyclohexylmethyl)[(4-phenylphenyl) methyl] sulfamoyl] benzoic acid) with an improved IC50 of 0.8 mu M on human mPGES-1. For the most promising compounds a broad pharmacological characterization has been carried out to estimate their anti-inflammatory potential. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2013.10.006
-
作为产物:描述:参考文献:名称:Heucke, Justus Liebigs Annalen der Chemie, 1889, vol. 255, p. 191摘要:DOI:
文献信息
-
Pt-Sn/γ-Al2O3-Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines作者:Wei He、Liandi Wang、Chenglin Sun、Kaikai Wu、Songbo He、Jiping Chen、Ping Wu、Zhengkun YuDOI:10.1002/chem.201101725日期:2011.11.18syntheses of secondary and tertiary amines by highly efficient direct N‐alkylation of primary and secondary amines with alcohols or by deaminative self‐coupling of primary amines have been successfully realized by means of a heterogeneous bimetallic Pt–Sn/γ‐Al2O3 catalyst (0.5 wt % Pt, Pt/Sn molar ratio=1:3) through a borrowing‐hydrogen strategy. In the presence of oxygen, imines were also efficiently prepared多功能通过高效直接伯和仲胺与醇或由deaminative自偶联的伯胺的已成功由多相双金属的Pt-Sn系来实现/γ-Al系的N-烷基化仲胺和叔胺的合成2 ö 3催化剂(0.5 wt%Pt,Pt / Sn摩尔比= 1:3)通过借位氢策略。在氧气存在下,还可以通过胺与醇或两种伯胺之间的串联反应有效地制备亚胺。所提出的机理表明,醇或胺底物最初会脱氢成醛/酮或NH-亚胺,并同时形成[PtSn]氢化物。醛/酮物种的缩合或NH-亚胺中间体与另一种胺分子的脱氨基反应形成N-取代的亚胺,然后在氮气氛下原位生成的[PtSn]氢化物将其还原为新的胺产物最终产品在氧气气氛下保持不变。将Pt-Sn系/γ-Al系2 ö 3 该催化剂可轻松回收利用,而无需进行Pt金属的浸出,并且对多种胺和醇类底物表现出非常高的催化活性,这表明其在直接生产仲胺和叔胺以及N-取代的亚胺中的应用潜力。
-
Reduction and Benzylation by Means of Benzyl Alcohol. II. N-Benzylation. The Preparation of Secondary Aromatic Benzylamines作者:Yaër SprinzakDOI:10.1021/ja01594a064日期:1956.7
-
Stabilized polyolefin compositions申请人:Kumar Vijayendra公开号:US20070149660A1公开(公告)日:2007-06-28Disclosed are compositions comprising antioxidants and stabilizers, such as, acid scavengers or organic phosphorus stabilizers, and optionally further comprising co-stabilizers. The disclosed compositions are useful as stabilizers for polyolefins and other polymeric materials. The disclosed compositions and methods generally provide longer shelf lifes and better oxidative resistance to materials than currently available antioxidants.
-
Lubricant oil compositions申请人:Cholli L. Ashok公开号:US20070161522A1公开(公告)日:2007-07-12Compositions comprise first antioxidants and first additives, such as, a surface additives, performance enhancing additives and lubricant protective additives and optionally second additives and/or second antioxidants. The compositions are useful to improve lubricants, lubricant oils and other lubricant materials. The compositions and methods generally provide longer shelf lives, increased oxidative resistance, improved quality and/or enhanced performance to lubricants or lubricant oils.
-
Lubricant Oil Compositions申请人:Cholli Ashok L.公开号:US20120004150A1公开(公告)日:2012-01-05Compositions comprise first antioxidants and first additives, such as, a surface additives, performance enhancing additives and lubricant protective additives and optionally second additives and/or second antioxidants. The compositions are useful to improve lubricants, lubricant oils and other lubricant materials. The compositions and methods generally provide longer shelf lives, increased oxidative resistance, improved quality and/or enhanced performance to lubricants or lubricant oils.
表征谱图
-
氢谱1HNMR
-
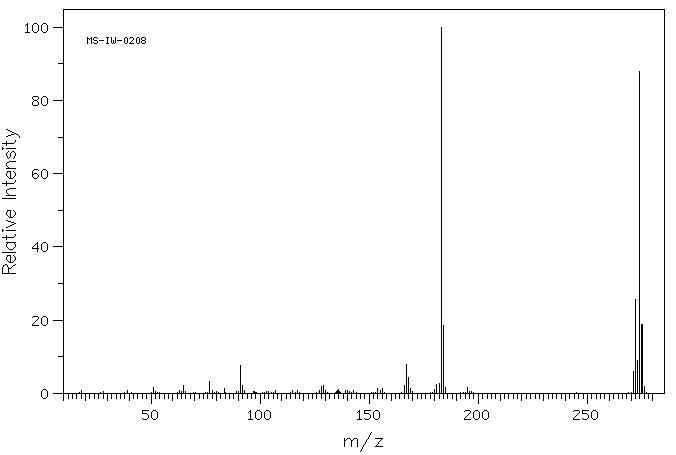
质谱MS
-
碳谱13CNMR
-
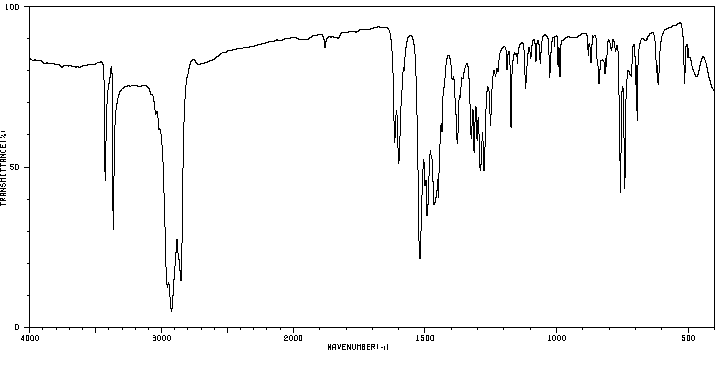
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫