1,2-dihydroxy-3,4-dimethoxy-6-methylbenzene | 54826-79-8
中文名称
——
中文别名
——
英文名称
1,2-dihydroxy-3,4-dimethoxy-6-methylbenzene
英文别名
2,3-dihydroxy-4,5-dimethoxytoluene;3,4-dihydroxy-1,2-dimethoxy-5-methylbenzene;3,4-dimethoxy-6-methyl-pyrocatechol;3,4-Dimethoxy-6-methyl-brenzcatechin;2,3-Dihydroxy-4,5-dimethoxy-toluol;3,4-Dimethoxy-6-methylpyrocatechol;3,4-dimethoxy-6-methylbenzene-1,2-diol
CAS
54826-79-8
化学式
C9H12O4
mdl
——
分子量
184.192
InChiKey
DNTPARUYPAHKHN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:101 °C
-
沸点:337.5±37.0 °C(Predicted)
-
密度:1.223±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:58.9
-
氢给体数:2
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4,5-三甲氧基甲苯 3,4,5-trimethoxytoluene 6443-69-2 C10H14O3 182.219 3.4-二甲氧基-2-羟基-6-甲基苯乙酮 1-(2-hydroxy-3,4-dimethoxy-6-methyl-phenyl)-ethanone 63542-37-0 C11H14O4 210.23
反应信息
-
作为反应物:描述:1,2-dihydroxy-3,4-dimethoxy-6-methylbenzene 在 vanadia sodium chlorate 、 硫酸 作用下, 以 水 为溶剂, 反应 6.0h, 以88%的产率得到烟曲霉醌参考文献:名称:Synthesis and Spectroscopic Characterization of [1′-14C]Ubiquinone-2, [1′-14C]-5-Demethoxy-5-hydroxyubiquinone-2, and [1′-14C]-5-Demethoxyubiquinone-2摘要:DOI:10.1002/1099-0690(200209)2002:17<3015::aid-ejoc3015>3.0.co;2-g
-
作为产物:描述:3,4,5-三甲氧基甲苯 在 sodium hydroxide 、 三氯化铝 、 乙醚 、 双氧水 作用下, 生成 1,2-dihydroxy-3,4-dimethoxy-6-methylbenzene参考文献:名称:122. 1:2:3:4-四羟基苯的衍生物。第七部分 熏蒸素的合成摘要:DOI:10.1039/jr9410000670
文献信息
-
A Penicillium sp. F33 metabolite and its synthetic derivatives inhibit acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (a key enzyme in platelet-activating factor biosynthesis) and carrageenan-induced paw edema in mice作者:Yasuhiro Yamazaki、Kengo Yasuda、Tensei Matsuyama、Takuya Ishihara、Ryoko Higa、Taira Sawairi、Masahiko Yamaguchi、Masahiro Egi、Shuji Akai、Toshio Miyase、Akira Ikari、Masao Miwa、Junko SugataniDOI:10.1016/j.bcp.2013.06.021日期:2013.9Acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine (lyso-PAF) acetyltransferase is a key enzyme in the biosynthesis of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) in inflammatory cells. Substances which inhibit this enzyme are of therapeutic interest. In this study, we screened for new inhibitors of lyso-PAF acetyltransferase with anti-inflammatory effects. In a metabolite from Penicillium sp. F33, we isolated an acetyltransferase inhibitor identified as dihydrofumigatin (2-methoxy-1,3,4-trihydroxy-5-methylbenzene) from high resolution mass spectrometer and NMR data. Dihydrofumigatin had strong acetyltransferase inhibitory activity, but was not stable in aqueous solution. Thus, we chemically synthesized its oxidized form fumigatin (3-hydroxy-2-methoxy-5-methyl-1,4-benzoquinone) and derivatives thereof, and evaluated their inhibitory effects. Strong inhibitory activity was observed for saturated fatty acid esters of fumigatin; the order of inhibition was 3-decanoyloxy-2-methoxy-5-methyl-1,4-benzoquinone (termed FUD-7, IC50 = 3 mu M) > 2-methoxy-5-methyl-3-tetradecanoyloxy-1,4-benzoquinone (termed FUD-8, IC50 = 20 mu M) > 3-hexanoyloxy-2-methoxy-5-methyl-1,4-benzoquinone (IC50 = 139 mu M). Interestingly, these compounds also significantly suppressed the gene expression of lyso-PAF acetyltransferase/LPCAT2 in mouse bone marrow-derived macrophages stimulated by lipopolysaccharide (LPS). We further evaluated the effect of these substances on anti-inflammatory activity in vivo using the carrageenan-induced mouse paw edema test. FUD-7 and FUD-8 at 2.5 mg/kg showed significant, 47.9-51.7%, inhibition stronger than that of prednisolone at 10 mg/kg (41.9%). These results suggest that FUD-7 and FUD-8 are potent inhibitors with anti-inflammatory activity. (C) 2013 Elsevier Inc. All rights reserved.
-
329. New synthesis of fumigatin, spinulosin monomethyl ethers, and aurantiogliocladin作者:T. R. Seshadri、G. B. VenkatasubramanianDOI:10.1039/jr9590001660日期:——
-
Holton, Dolores M.; Murphy, David, Journal of the Chemical Society. Perkin transactions II, 1980, p. 1757 - 1760作者:Holton, Dolores M.、Murphy, DavidDOI:——日期:——
-
Compositions and Methods With Enhanced Therapeutic Activity申请人:Chaplin David公开号:US20090137687A1公开(公告)日:2009-05-28This invention relates to novel tricyclic quinone and catechol compositions, compositions containing prodrugs of tricyclic quinone and catechol compositions, and methods of use for the treatment of solid tumor cancers and other vascular proliferative disorders. In certain aspects, the compositions of the invention are capable of generating both a vascular targeting effect and tumor cell cytotoxicity (e.g., by oxidative stress) in order to achieve an enhanced anti-tumor response in a patient.
表征谱图
-
氢谱1HNMR
-
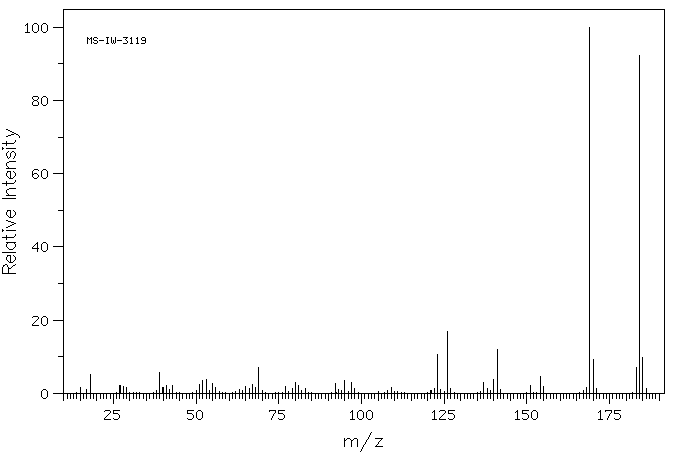
质谱MS
-
碳谱13CNMR
-
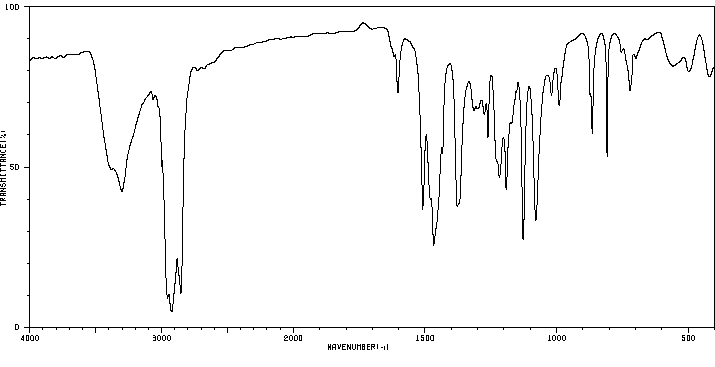
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚