ethyl (E)-2-(2-(2-chlorophenyl)hydrazono)propanoate | 43142-70-7
中文名称
——
中文别名
——
英文名称
ethyl (E)-2-(2-(2-chlorophenyl)hydrazono)propanoate
英文别名
2-(2-chloro-phenylhydrazono)-propionic acid ethyl ester;2-(2-Chlor-phenylhydrazono)-propionsaeure-aethylester;Brenztraubensaeureaethylester-(2-chlor-phenylhydrazon);(E)-Ethyl-pyruvat-2-chlorphenylhydrazon;Propanoic acid, 2-(2-chlorophenylhydrazono)-, ethyl ester;ethyl (2E)-2-[(2-chlorophenyl)hydrazinylidene]propanoate
CAS
43142-70-7
化学式
C11H13ClN2O2
mdl
——
分子量
240.689
InChiKey
HWZBVPRPFNROEW-MDWZMJQESA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:319.5±44.0 °C(Predicted)
-
密度:1.19±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:16
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:50.7
-
氢给体数:1
-
氢受体数:4
SDS
反应信息
-
作为反应物:描述:ethyl (E)-2-(2-(2-chlorophenyl)hydrazono)propanoate 在 喹啉 、 氢氧化钾 、 PPA 、 copper oxide-chromium oxide 作用下, 生成 7-氯吲哚参考文献:名称:Bz取代的吲哚和色氨酸合成实验。第三部分 四个Bz-氯吲哚和-色氨酸的合成摘要:DOI:10.1039/jr9550003499
-
作为产物:参考文献:名称:3取代的1H-吲哚-2-羧酸衍生物作为一类新的CysLT1选择性拮抗剂的发现。摘要:吲哚衍生物3-((E)-3-((3-((E)-2-(7-氯喹啉-2基)乙烯基)苯基)氨基)-3-氧代丙-1-烯-1-基) -7-甲氧基-1H-吲哚-2-羧酸(17k)被确定为一种新型且高效的选择性CysLT1拮抗剂,CysLT1和CysLT2的IC50值为0.0059 +/- 0.0011和15 +/- 4μM,分别。DOI:10.1021/acsmedchemlett.5b00482
文献信息
-
Fischer Indolization of Variously ortho-Substituted Phenylhydrazones. Fischer Indolization and Its Related Compounds. XXV.作者:Yasuoki MURAKAMI、Toshiko WATANABE、Yuusaku YOKOYAMA、Junko NAOMACHI、Haruo IWASE、Noriko WATANABE、Michiyo MORIHATA、Naomi OKUYAMA、Hiroyuki KAMAKURA、Tomoko TAKAHASHI、Hideko ATODA、Toshiaki TOJO、Kenji MORITA、Hisashi ISHIIDOI:10.1248/cpb.41.1910日期:——Various kinds of ethyl pyruvate 2-(2-substituted phenyl)hydrazones (1) were subjected to Fischer indolization with acid catalysts. All the phenylhydrazones gave corresponding normal 7-substituted indoles (2). In addition, phenyl-hydrazones whose ortho-substituent is electron-donative or has a central atom with an unshared electron pair tended to give the 4- or 5-substituted indole (3), which was produced by migration of the ortho-substituent during cyclization, whereas those whose ortho-substituent is electron-attractive tended to give little or no such abnormal product. The kind of acid catalyst used had some effect on the yield ratio of products but not on the kind of products.
-
Indoline analogs of idazoxan: potent .alpha.2-antagonists and .alpha.1-agonists作者:Gay P. Fagan、Christopher B. Chapleo、Anthony C. Lane、Malcolm Myers、Alan G. Roach、Colin F. C. Smith、Michael R. Stillings、Anthony P. WelbournDOI:10.1021/jm00400a009日期:1988.5The synthesis and alpha-adrenergic activity of a series of substituted 2-imidazolinylindolines are described. Substitution in the indoline ring generated compounds with a spectrum of adrenoceptor antagonist/agonist profiles that proved sensitive to both the nature and position of the substituent. Many of the derivatives possess greater presynaptic antagonist potency than the corresponding benzodioxan 1, dihydrobenzofuran 2, and indan 3 analogues; however, this alpha 2-antagonism is often accompanied by alpha 1-agonist activity. It was not possible to separate alpha 2-antagonist from alpha 1-agonist properties in this series. Compounds of most interest proved to be the N-ethyl 6, 5-chloro-N-methyl 18, and 5-chloro-N-ethyl 23 derivatives, all being potent alpha 2-antagonists and alpha 1-agonists. Substitution at the 4- and 7-position of the indoline ring generally gave compounds with nonselective agonist properties.
-
Hewitt, Journal of the Chemical Society, 1893, vol. 63, p. 871作者:HewittDOI:——日期:——
-
N-Benzylindole-2-carboxylic acids: potent functional antagonists of the CCR2b chemokine receptor作者:Jason G. Kettle、Alan W. Faull、Andy J. Barker、D.Huw Davies、Michael A. StoneDOI:10.1016/j.bmcl.2003.10.049日期:2004.1Screening of the corporate database led to the discovery of a novel series of N-benzylindole-2-carboxylic acid CCR2b chemokine receptor antagonists. These compounds demonstrate high affinity and functional inhibition of the CCR2b receptor. A discussion of the structure-activity relationships is presented, together with evidence for a highly selective receptor binding profile. (C) 2003 Elsevier Ltd. All rights reserved.
-
Murakami, Yasuoki; Yokoyama, Yuusaku; Miura, Tomoko, Heterocycles, 1984, vol. 22, # 5, p. 1211 - 1216作者:Murakami, Yasuoki、Yokoyama, Yuusaku、Miura, Tomoko、Hirasawa, Hiroko、Kamimura, Yuuko、Izaki, MiyakoDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
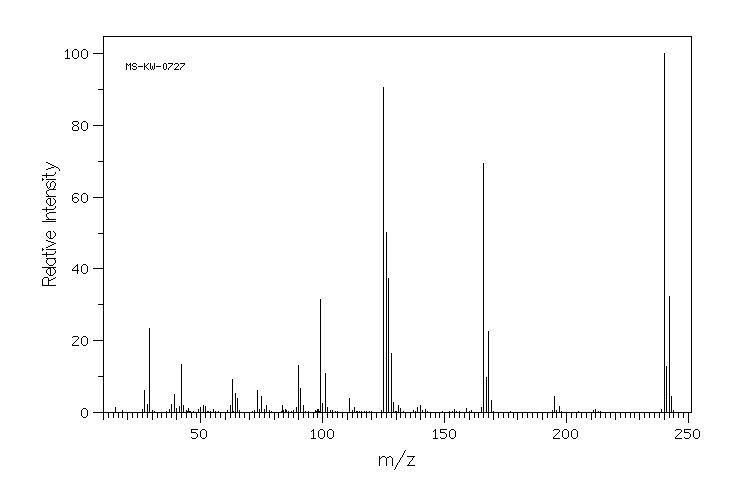
质谱MS
-
碳谱13CNMR
-
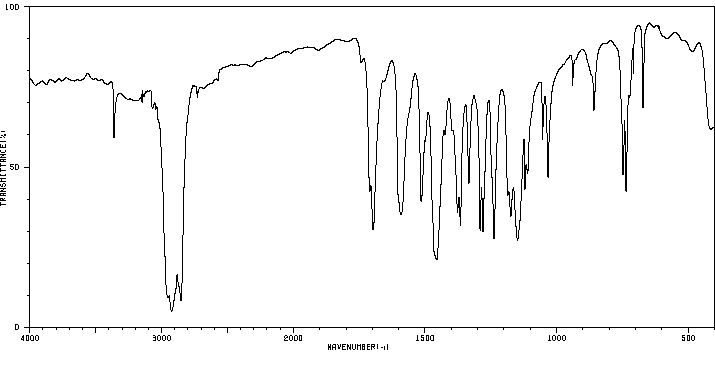
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫