2-氨基-4-(4-联苯基)噻唑 | 2834-79-9
中文名称
2-氨基-4-(4-联苯基)噻唑
中文别名
4-联苯-4-噻唑-2-胺;2-氨基-4-(对联苯)噻唑
英文名称
4-(biphenyl-4-yl)-1,3-thiazol-2-amine
英文别名
4-([1,1'-biphenyl]-4-yl)thiazol-2-amine;4-(biphenyl-4-yl)thiazol-2-amine;2-Amino-4-p-biphenylyl-thiazol;2-Amino-4-(4-biphenylyl)thiazole;4-(4-phenylphenyl)-1,3-thiazol-2-amine
CAS
2834-79-9
化学式
C15H12N2S
mdl
MFCD00047059
分子量
252.34
InChiKey
HTAUVJPDFDVVHV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:207-208 °C
-
沸点:489.0±14.0 °C(Predicted)
-
密度:1.230±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
海关编码:2934100090
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:密封避光保存。
SDS
制备方法与用途
制备方法:有机合成。
用途简介: 暂无内容
用途: 有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-4-(4-溴苯基)噻唑 4-(4-bromophenyl)-1,3-thiazol-2-amine 2103-94-8 C9H7BrN2S 255.138
反应信息
-
作为反应物:描述:参考文献:名称:一种快速、温和、高效的 2-氨基噻唑 C-5 碘化/硫氰化方法摘要:图形摘要 摘要 开发了一种高效、绿色、快速的多任务协议,用于使用碘酸和 PEG-400 水溶液选择性地 C-5 取代 2-氨基噻唑。该方法发现适用于 C-5 取代,即分别使用碘和硫氰酸铵对 2-氨基噻唑进行碘化和硫氰化。碘酸被发现是一种良好的氧化剂,PEG-400 水溶液作为绿色反应溶剂。DOI:10.1080/10426507.2016.1149851
-
作为产物:描述:参考文献:名称:NBS和硫脲通过炔烃的一锅法转化2-氨基-4-芳基噻唑摘要:通过NBS处理,各种烷基芳烃成功地转化为相应的2-氨基-4-芳基噻唑和2,4-二芳基噻唑,然后与硫脲或芳硫代酰胺反应。DOI:10.1002/ejoc.201900100
文献信息
-
Inhibitors of histone deacetylase申请人:——公开号:US20020177594A1公开(公告)日:2002-11-28Compounds having the formula 1 or therapeutically acceptable salts thereof, are histone deacetylase (HDAC) inhibitors. Preparation of the compounds, compositions containing the compounds, and treatment of diseases using the compounds are disclosed.
-
N-(5-MEMBERED AROMATIC RING)-AMIDO ANTI-VIRAL COMPOUNDS申请人:Schmitz Ulrich Franz公开号:US20070265265A1公开(公告)日:2007-11-15Disclosed are compounds having Formula (I) and the compositions and methods thereof for treating or preventing a viral infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, R 2 , m, R, V, W, T, Z, R 1 , Y, and p are disclosed herein.揭示了具有Formula (I)的化合物,以及用于治疗或预防由Flaviviridae病毒家族中的病毒至少部分介导的病毒感染的组合物和方法,其中A、R2、m、R、V、W、T、Z、R1、Y和p在此处被揭示。
-
Synthesis, activity, and docking study of phenylthiazole acids as potential agonists of PPARγ作者:Liang Ma、Taijin Wang、Min Shi、Haoyu YeDOI:10.2147/dddt.s106406日期:——glucose and lipid homeostasis, and PPARγ ligands possess therapeutic potential in these as well as other areas. In this study, a series of phenylthiazole acids have been synthesized and evaluated for agonistic activity by a convenient fluorescence polarization-based PPARγ ligand screening assay. Compound 4t, as a potential PPARγ agonist with half maximal effective concentration (EC50) 0.75±0.20 μM, exhibited
-
Convenient and simple synthesis of 2-aminothiazoles by the reaction of α-halo ketone carbonyls with ammonium thiocyanate in the presence of N-methylimidazole作者:H.M. Meshram、Pramod B. Thakur、B. Madhu Babu、Vikas M. BangadeDOI:10.1016/j.tetlet.2012.07.080日期:2012.9Substituted 2-aminothiazole derivatives were obtained as a result of N-methylimidazole catalyzed cyclization of α-halo ketone carbonyls with ammonium thiocyanate in water–alcoholic media. The generality of the method has been demonstrated by screening a series of aromatic/heteroaromatic/aliphatic α-halo ketones, α-halo β-diketones, and α-halo β-ketoesters. The developed method is simple, mild, and
-
I2/CuO-catalyzed tandem cyclization strategy for one-pot synthesis of substituted 2-aminothiozole from easily available aromatic ketones/α,β-unsaturated ketones and thiourea作者:Yan-Ping Zhu、Jing-Jing Yuan、Qin Zhao、Mi Lian、Qing-He Gao、Mei-Cai Liu、Yan Yang、An-Xin WuDOI:10.1016/j.tet.2011.10.074日期:2012.1A concise and efficient one-pot process from easily available methyl ketones/unsaturated methyl ketones and thiourea was developed for the synthesis of 2-aminothiazoles under the media of I2/CuO. The method can highly stereoselectivity obtain the E-isomers of 4-ethenyl-2-aminothiazoles (5a–f). All these target molecules were characterized by NMR, HRMS and IR spectra. Furthermore, the target compounds
表征谱图
-
氢谱1HNMR
-
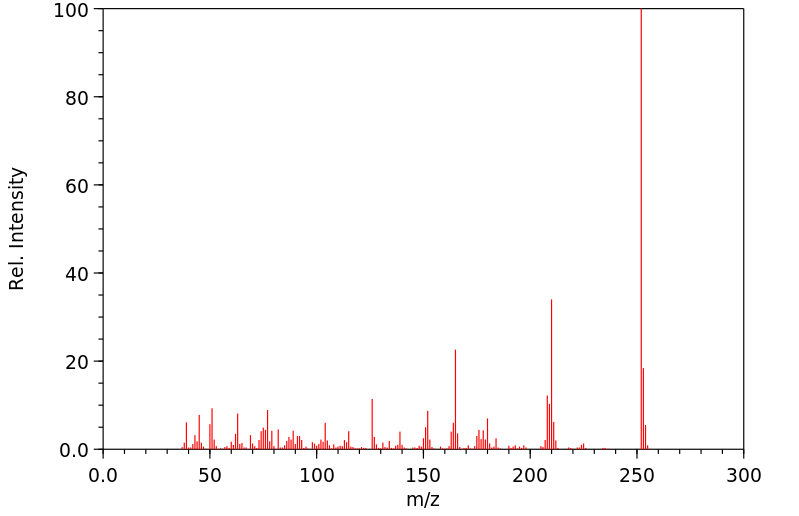
质谱MS
-
碳谱13CNMR
-
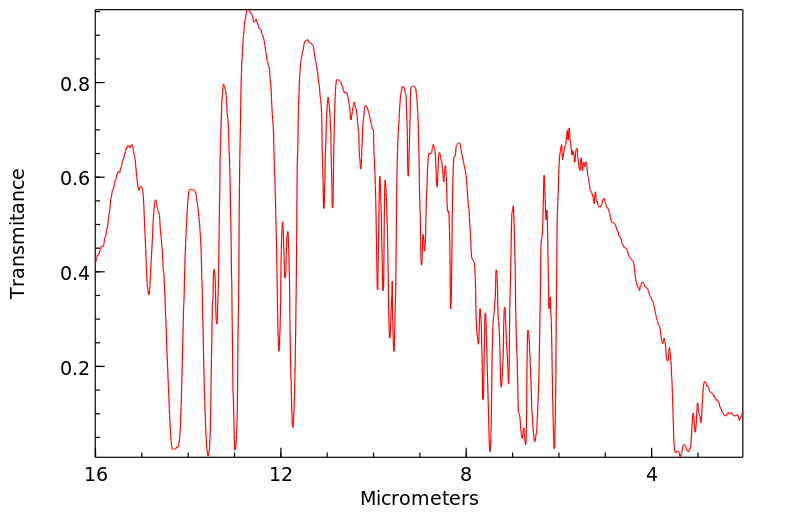
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫