2-氨基对甲酚盐酸盐 | 2977-71-1
中文名称
2-氨基对甲酚盐酸盐
中文别名
2-氨基-4-甲基苯酚盐酸盐
英文名称
p-methyl-o-aminophenol hydrochloride
英文别名
2-amino-p-cresol hydrochloride;2-amino-4-methylphenol hydrochloride;4-methyl-2-aminophenol hydrochloride;2-amino-4-methylphenol;hydron;chloride
CAS
2977-71-1
化学式
C7H9NO*ClH
mdl
——
分子量
159.615
InChiKey
MZMUKGFYWNOPAU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:46.2
-
氢给体数:3
-
氢受体数:2
安全信息
-
安全说明:S16,S26,S36,S37,S39,S45
-
危险类别码:R23/24/25,R36/37/38
-
海关编码:2922299090
SDS
2-氨基对甲酚盐酸盐 修改号码:5
模块 1. 化学品
产品名称: 2-Amino-p-cresol Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第5级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吞咽可能有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-氨基对甲酚盐酸盐
2-氨基对甲酚盐酸盐 修改号码:5
模块 3. 成分/组成信息
百分比: >98.0%(T)
CAS编码: 2977-71-1
俗名: 2-Amino-4-methylphenol Hydrochloride , 2-Hydroxy-5-methylaniline
Hydrochloride
分子式:
C7H9NO·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
2-氨基对甲酚盐酸盐 修改号码:5
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-红灰色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
2-氨基对甲酚盐酸盐 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Amino-p-cresol Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第5级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吞咽可能有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-氨基对甲酚盐酸盐
2-氨基对甲酚盐酸盐 修改号码:5
模块 3. 成分/组成信息
百分比: >98.0%(T)
CAS编码: 2977-71-1
俗名: 2-Amino-4-methylphenol Hydrochloride , 2-Hydroxy-5-methylaniline
Hydrochloride
分子式:
C7H9NO·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
2-氨基对甲酚盐酸盐 修改号码:5
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-红灰色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
2-氨基对甲酚盐酸盐 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:2-氨基对甲酚盐酸盐 在 三乙胺 、 tin(ll) chloride 、 亚硝酸异戊酯 作用下, 以 二氯甲烷 为溶剂, 反应 1.5h, 生成 (E)-ethyl pyruvate 2-<2-(methanesulfonyloxy)-4-methyl>phenylhydrazone参考文献:名称:Substituent Effects in the Fischer Indolization of (2-Sulfonyloxyphenyl)hydrazones (Fischer Indolization and Related Compounds. XXX).摘要:研究了丙酮酸乙酯 2-(2-甲磺酰氧基)苯基腙(7)的各种附加取代基在费舍尔吲哚化反应中的影响,当取代基为 3-、4-或 5-位的甲基时,正常 7-甲磺酰氧基吲哚的产量取决于位置。在靠近甲烷磺酰氧基的位置上具有甲基的酰肼往往能生成正常的 7-甲烷磺酰氧基吲哚,其生成量占吲哚类总产物的比例较高。在一系列 4-取代苯基肼(7d、e、f)中,与提供电子的取代基相比,提供更多电子的取代基倾向于生成正常的 7-甲烷磺酰氧基吲哚,在吲哚类总产物中所占的比例更高。在 4 位(7f)上具有强夺电子取代基的腙发生了分子内芳香亲核取代烯肼的反应,生成了噌啉衍生物。DOI:10.1248/cpb.47.791
文献信息
-
Facile synthesis of novel nonpeptide angiotensin II receptor antagonists作者:Ling-Chun Yang、Guan-Xin Zhang、Nan-Zhi Zou、Chuan-Min QiDOI:10.1002/jhet.5570400623日期:2003.11A series of Losartan analogues 1-12 with different functional groups were synthesized and characterized. In comparison with the previous reports, the synthetic procedures described in this paper have been optimized as follows below: 1) preparation of aromatic amine 15 and 18 through hydrogenation by employing Raney Ni and hydrazine as catalysts in place of palladium; 2) preparation of nitro-compound
-
Base catalysed rearrangements involving ylide intermediates. Part 13. Further rearrangements of 2-oxidoanilinium ylides作者:W. David Ollis、Ratnasamy Somanathan、Ian O. SutherlandDOI:10.1039/p19810002930日期:——The 2-oxidoanilinium ylides (7) rearrange on heating to give the ethers (10) together with the dienones (8) and the phenols (11) for cases where the aromatic ring has a 5-alkyl substituent or a mixture of the dienones (8) and (12) for cases where the aromatic ring is 3,5-disubstituted. A study of the deuteriated ylides (25) shows that these reactions involve competing concerted and radical-pair processes
-
Metal-free direct annulation of 2-aminophenols and 2-aminothiophenols with unactivated amides through transamidation: Access to polysubstituted benzoxazole and benzothiazole derivatives作者:Vishal Kumar、Sanjeev Dhawan、Renu Bala、Pankaj Sanjay Girase、Parvesh Singh、Rajshekhar KarpoormathDOI:10.1016/j.tet.2022.132794日期:2022.6hydrochloride played a crucial role in amide activation followed nucleophilic attack that through eliminations of amine as a side product, and de-hydrolysis step leads to final annulation. This versatile strategy is applicable to a wide variety of differently substituted o-aminophenols, unactivated aliphatic and aromatic amides, yielding the corresponding product in good to excellent yields in a single step
-
Noelting; Kohn, Chemische Berichte, 1884, vol. 17, p. 363作者:Noelting、KohnDOI:——日期:——
-
Hofmann,A. W.; v. Miller, Chemische Berichte, 1881, vol. 14, p. 572作者:Hofmann,A. W.、v. MillerDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
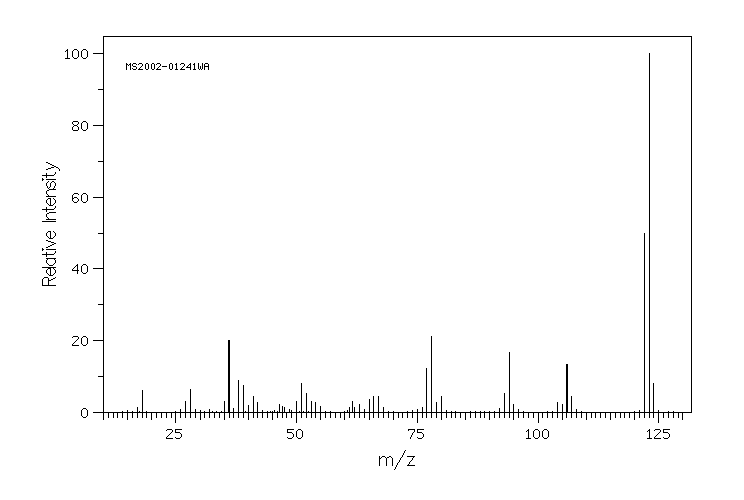
质谱MS
-
碳谱13CNMR
-
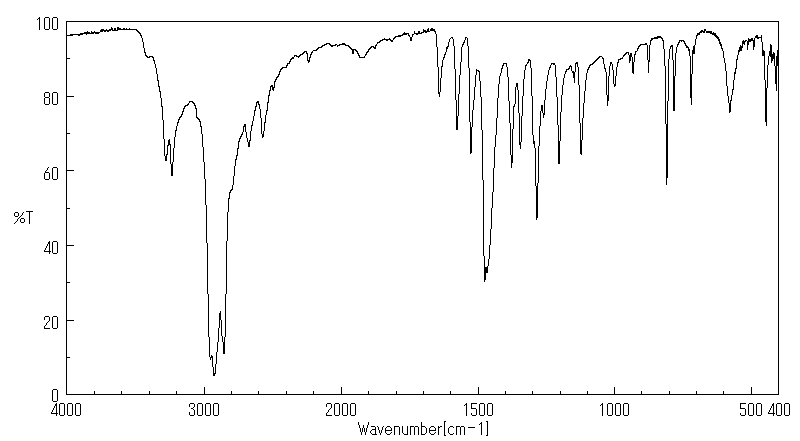
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚