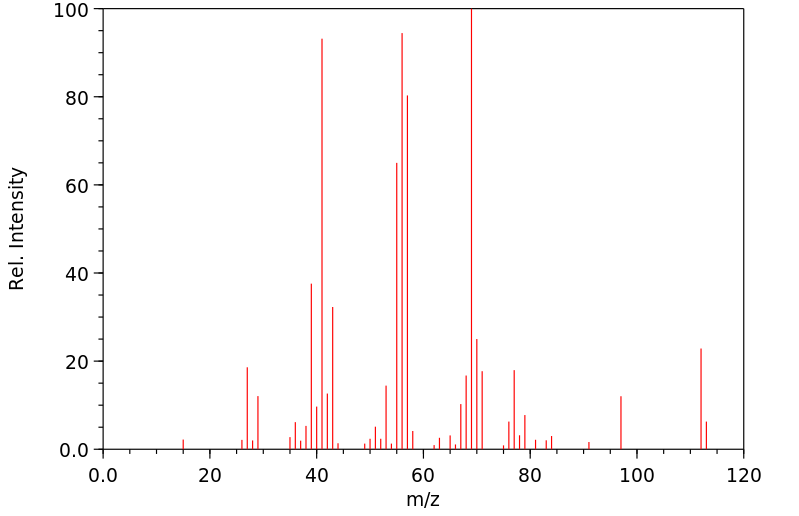
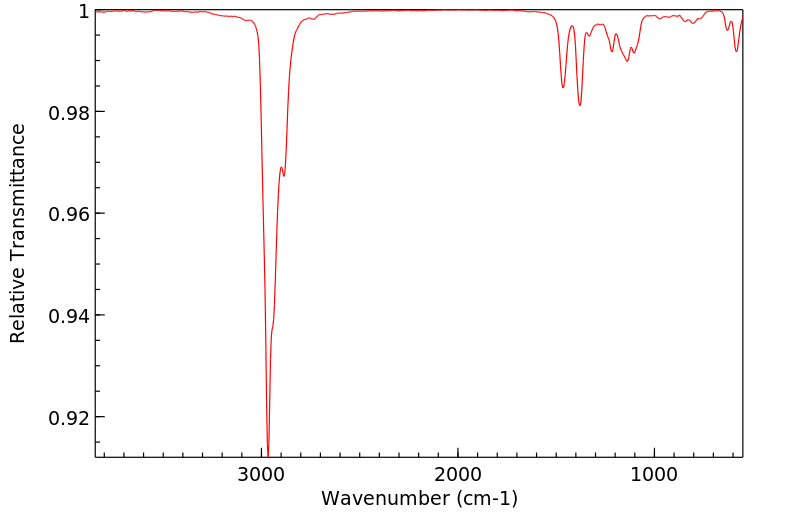
2-氯-2,5-二甲基己烷 | 29342-44-7
中文名称
2-氯-2,5-二甲基己烷
中文别名
——
英文名称
2-Chlor-2,5-dimethylhexan
英文别名
2-chloro-2,5-dimethylhexane
CAS
29342-44-7
化学式
C8H17Cl
mdl
MFCD00060775
分子量
148.676
InChiKey
SVRDRJZPEYTXKX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-41.9°C (estimate)
-
沸点:98 °C / 150mmHg
-
密度:0.86
-
闪点:52 °C
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,也没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
海关编码:2903199000
SDS
2-Chloro-2,5-dimethylhexane
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Chloro-2,5-dimethylhexane
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Chloro-2,5-dimethylhexane
Percent: >98.0%(GC)
CAS Number: 29342-44-7
Chemical Formula: C8H17Cl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2-Chloro-2,5-dimethylhexane
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
2-Chloro-2,5-dimethylhexane
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 98°C/20kPa
Flash point: 52°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.86
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
2-Chloro-2,5-dimethylhexane
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 1993
Proper shipping name: Flammable liquid, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Chloro-2,5-dimethylhexane
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Chloro-2,5-dimethylhexane
Percent: >98.0%(GC)
CAS Number: 29342-44-7
Chemical Formula: C8H17Cl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2-Chloro-2,5-dimethylhexane
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
2-Chloro-2,5-dimethylhexane
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 98°C/20kPa
Flash point: 52°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.86
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Spark, Open flame, Static discharge
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
2-Chloro-2,5-dimethylhexane
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 1993
Proper shipping name: Flammable liquid, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:2-氯-2,5-二甲基己烷 在 双氧水 作用下, 反应 20.0h, 以75%的产率得到2-Hydroperoxy-2,5-dimethylhexane参考文献:名称:Arakelyan, A. S.; Dvoryanchikov, A. I.; Gevorkyan, A. A., Journal of Organic Chemistry USSR (English Translation), 1987, vol. 23, # 11, p. 2047 - 2050摘要:DOI:
-
作为产物:参考文献:名称:Schneider, Hans-Joerg; Philippi, Klaus, Journal of Chemical Research, Miniprint, 1984, # 4, p. 901 - 951摘要:DOI:
文献信息
-
Alkyllithium Compounds Bearing Electrophilic Functional Groups: A Flash Chemistry Approach作者:Aiichiro Nagaki、Hiroki Yamashita、Katsuyuki Hirose、Yuta Tsuchihashi、Jun‐ichi YoshidaDOI:10.1002/anie.201814088日期:2019.3.18Flash chemistry based on flow microreactor systems allowed alkyllithiums bearing electrophilic functional groups to be successfully generated and used for subsequent reactions. The series of reactions with high reactivity was achieved by extremely accurate control over residence time in a controlled and selective manner.
-
Optically active bisoxazoline compounds, process for production of the same and use thereof申请人:Itagaki Makoto公开号:US20060149077A1公开(公告)日:2006-07-06Optically active bisoxazoline compounds represented by the general formula (1), a process for producing the compounds, and a process for producing cyclopropanecarboxylic esters by using the same: wherein R 1 and R 2 are the same and each represents C 1-6 alkyl, substituted or unsubstituted aralkyl, or substituted or unsubstituted phenyl or R 1 and R 2 are bonded each other together with the carbon atom of oxazoline ring to which they are bonded to form a ring; R 3 is substituted or unsubstituted naphthyl; R 4 and R 5 are the same and each represent hydrogen or C 1-6 alkyl, or R 4 and R 5 are bonded each other together with the carbon atom to which they are bonded to form a cycloalkyl ring having 3 to 6 carbon atoms; and * represents an asymmetric center.
-
OPTICALLY ACTIVE BISOXAZOLINE COMPOUNDS, PROCESS FOR PRODUCTION OF THE SAME AND USE THEREOF申请人:ITAGAKI Makoto公开号:US20080076941A1公开(公告)日:2008-03-27Optically active bisoxazoline compounds represented by the general formula (1), a process for producing the compounds, and a process for producing cyclopropanecarboxylic esters by using the same: wherein R 1 and R 2 are the same and each represents C 1-6 alkyl, substituted or unsubstituted aralkyl, or substituted or unsubstituted phenyl or R 1 and R 2 are bonded each other together with the carbon atom of oxazoline ring to which they are bonded to form a ring; R 3 is substituted or unsubstituted naphthyl; R 4 and R 5 are the same and each represent hydrogen or C 1-6 alkyl, or R 4 and R 5 are bonded each other together with the carbon atom to which they are bonded to form a cycloalkyl ring having 3 to 6 carbon atoms; and * represents an asymmetric center.
-
Substituierte Phenylpropylhalogenide, ihre Herstellung und Verwendung申请人:BASF Aktiengesellschaft公开号:EP0009077A1公开(公告)日:1980-04-02Neues Verfahren zur Herstellung von Phenylpropylhalogeniden der Formel durch Umsetzung des entsprechenden allein durch R2 und R' substituierten Phenylpropylhalogenids mit einem Alkohol, Alkylhalogenid der Olefin und Phenylpropylhalogenide, in denen R3 bestimmte Bedeutungen hat.一种制备式中苯基丙基卤化物的新工艺 将相应的仅被 R2 和 R' 取代的苯基丙基卤化物与醇、烯烃的烷基卤化物和苯基丙基卤化物(其中 R3 具有特定含义)反应。
-
OPTICALLY ACTIVE COPPER CATALYST COMPOSITION申请人:Sumitomo Chemical Company, Limited公开号:EP1607136A1公开(公告)日:2005-12-21There is provided an optically active copper catalyst composition comprising (a) an optically active salicylideneaminoalcohol represented by the formula (1): wherein R1 and R2 are the same or different, and independently represent a substituted or unsubstituted lower alkyl group, a substituted or unsubstituted aralkyl group, or a substituted or unsubstituted aryl group; X1 and X2 are the same or different, and independently represent a hydrogen atom, a lower alkoxy group, a nitro group, a lower alkoxycarbonyl group, a cyano group or a halogen atom; and * represents an asymmetric center, provided that both of X1 and X2 don't represent hydrogen atoms, (b) a monovalent or divalent copper compound, and (c-1) a lithium compound or (c-2) a compound selected from aluminum compounds having Lewis acidity, titanium compounds having Lewis acidity, boron compounds having Lewis acidity, zirconium compounds having Lewis acidity and hafnium compounds having Lewis acidity; and a process for producing an optically active cyclopropane compound by using the same.提供了一种光学活性铜催化剂组合物,其中包括 (a) 由式(1)代表的光学活性水杨基氨基醇: 其中 R1 和 R2 相同或不同,并各自代表取代或未取代的低级烷基、取代或未取代的芳烷基或取代或未取代的芳基; X1 和 X2 相同或不同,且各自代表氢原子、低级烷氧基、硝基、低级烷氧羰基、氰基或卤素原子; * 代表不对称中心,条件是 X1 和 X2 均不代表氢原子、 (b) 一价或二价铜化合物,以及 (c-1) 锂化合物或 (c-2) 选自具有路易斯酸性的铝化合物、具有路易斯酸性的钛化合物、具有路易斯酸性的硼化合物、具有路易斯酸性的锆化合物和具有路易斯酸性的铪化合物的化合物;以及利用上述化合物生产光学活性环丙烷化合物的工艺。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷