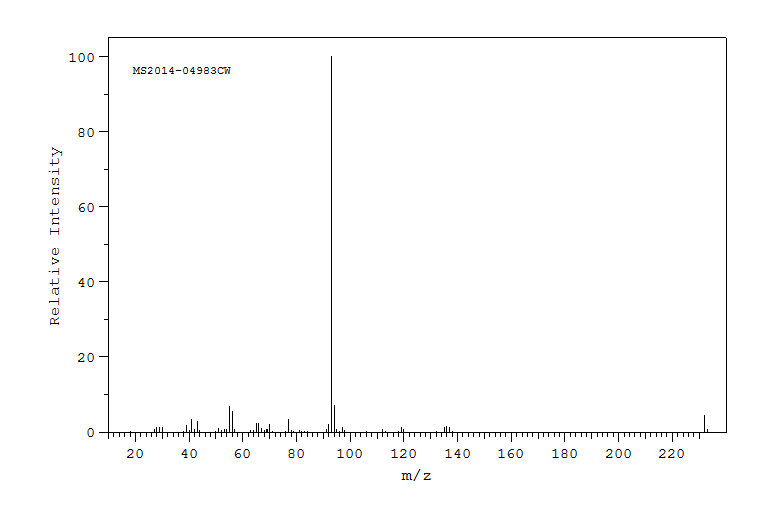
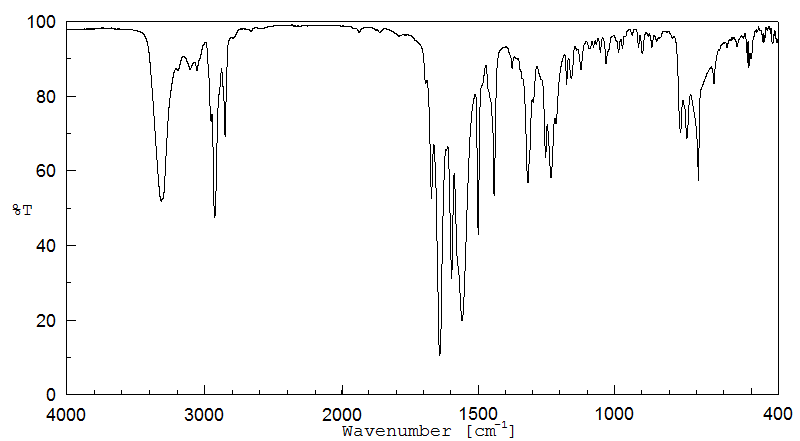
代谢
从接受2500 ppm 赛杜隆饮食的狗身上收集的尿液中,使用比色法、质谱、红外光谱和核磁共振光谱分析了母体化合物和代谢物,并提出了代谢途径;没有找到母体化合物,但分离并鉴定了三种羟基取代赛杜隆的葡萄糖苷酸结合物,一种在苯环上取代,一种在环己基环上取代,另一种在这两个位置上都取代;似乎赛杜隆首先在任一位置羟基化,然后这两个单羟基化合物再次羟基化形成1-(4-羟基-2-甲基环己基)-3-对羟基苯基脲;将狗的尿液与接受相似2500 ppm饮食的大鼠的尿液进行比较,发现大鼠形成的环己基取代化合物比狗略少;这两种动物尿液中结合的总赛杜隆代谢物为800-1000 ppm。
Urine from a dog receiving 2500 ppm siduron in the diet was collected and analyzed for the parent compound and metabolites using a colorimetric method, and mass, IR, and NMR spectrometry, and a metabolic pathway was proposed; none of the parent compound was found, but three glucuronide conjugates of hydroxysubstituted siduron were isolated and identified, one substituted on the phenyl ring, one substituted on the cyclohexyl ring, and another substituted in both locations; it appears that siduron is first hydroxylated at either location, then both of these mono-hydroxylated compounds are hydroxylated again to form 1-(4-hydroxy-2-methylcyclo-hexyl)-3-p-hydroxyphenylurea; comparison of urine from the dog with that from a rat on a similar 2500 ppm diet, showed that the rat formed slightly less of the cyclohexyl-substituted compound than the dog; 800-1000 ppm of total siduron metabolites was bound in the urine of these two animals.
来源:Hazardous Substances Data Bank (HSDB)