2-氯-5-(氟磺酰基)苯甲酸 | 21346-66-7
中文名称
2-氯-5-(氟磺酰基)苯甲酸
中文别名
——
英文名称
2-chloro-5-(fluorosulfonyl)benzoic acid
英文别名
2-chloro-5-(fluorosulpho)benzoic acid;2-chloro-5-fluorosulfonylbenzoic acid
CAS
21346-66-7
化学式
C7H4ClFO4S
mdl
MFCD00007418
分子量
238.624
InChiKey
HXVUCIYXBTYQLK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148-150 °C(lit.)
-
沸点:370.0±32.0 °C(Predicted)
-
密度:1.5768 (estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:14
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:79.8
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险等级:8
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2916399090
-
包装等级:III
-
危险品运输编号:UN 3261 8/PG 2
-
危险类别:8
-
危险性防范说明:P260,P264,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P363,P405,P501
-
危险性描述:H314
SDS
| Name: | 2-Chloro-5-(Fluorosulfonyl)-Benzoic Acid 99+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 21346-66-7 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 21346-66-7 | 2-Chloro-5-(Fluorosulfonyl)-Benzoic Ac | 99+ | 244-338-9 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 21346-66-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 148.00 - 150.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H4ClFO4S
Molecular Weight: 238.62
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, hydrogen sulfide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 21346-66-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Chloro-5-(Fluorosulfonyl)-Benzoic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.*
Hazard Class: 8
UN Number: 3261
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 21346-66-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 21346-66-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 21346-66-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-5-氯磺酰基苯甲酸 2-chloro-5-(chlorosulfonyl)benzoic acid 137-64-4 C7H4Cl2O4S 255.078 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Chloro-5-fluorosulfonylbenzoyl chloride 90929-66-1 C7H3Cl2FO3S 257.069 —— 2-Chlor-5-sulfino-benzoesaeure 89692-75-1 C7H5ClO4S 220.633 2-氯-5-(甲基磺酰基)苯甲酸 2-chloro-5-(methylsulfamoyl)benzoic acid 68901-09-7 C8H8ClNO4S 249.675
反应信息
-
作为反应物:描述:参考文献:名称:芳香族磺酰氟共价动力学稳定运甲状腺素蛋白以防止淀粉样蛋白形成,同时提供荧光缀合物摘要:选择性地结合给定蛋白质,然后进行快速化学选择性反应以形成共价缀合物的分子在药物开发中具有实用性。本文设想了一个 1,3,4-恶二唑库,其 2 位被芳基磺酰氟取代,5 位被取代的芳基取代,已知该芳基对转甲状腺素蛋白 (TTR) 的内部甲状腺素结合亚位点具有高亲和力基于结构的设计原理并通过化学合成。当结合在甲状腺素结合位点时,大多数芳基磺酰氟会与 pKa 扰动的 K15 残基发生快速且化学选择性的反应,从而在动力学上稳定 TTR,从而防止已知会导致多发性神经病的淀粉样原纤维形成。结合 t50 范围为 1 至 4 分钟,比甲状腺素结合位点外的水解反应快约 1400 倍。X 射线晶体学证实了预期的结合方向,并揭示了磺酰氟活化导致磺酰胺与 TTR 的连接。一些芳基磺酰氟在血浆中与 TTR 有效形成缀合物。合成的 11 种 TTR 共价动力学稳定剂在缀合时表现出荧光,因此由于发色团的环境敏感荧光而可能具有成像应用。DOI:10.1021/ja311729d
-
作为产物:描述:2-氯-5-氯磺酰基苯甲酸 在 potassium hydrogen bifluoride 作用下, 以 1,4-二氧六环 、 水 为溶剂, 反应 0.5h, 以98%的产率得到2-氯-5-(氟磺酰基)苯甲酸参考文献:名称:芳香族磺酰氟共价动力学稳定运甲状腺素蛋白以防止淀粉样蛋白形成,同时提供荧光缀合物摘要:选择性地结合给定蛋白质,然后进行快速化学选择性反应以形成共价缀合物的分子在药物开发中具有实用性。本文设想了一个 1,3,4-恶二唑库,其 2 位被芳基磺酰氟取代,5 位被取代的芳基取代,已知该芳基对转甲状腺素蛋白 (TTR) 的内部甲状腺素结合亚位点具有高亲和力基于结构的设计原理并通过化学合成。当结合在甲状腺素结合位点时,大多数芳基磺酰氟会与 pKa 扰动的 K15 残基发生快速且化学选择性的反应,从而在动力学上稳定 TTR,从而防止已知会导致多发性神经病的淀粉样原纤维形成。结合 t50 范围为 1 至 4 分钟,比甲状腺素结合位点外的水解反应快约 1400 倍。X 射线晶体学证实了预期的结合方向,并揭示了磺酰氟活化导致磺酰胺与 TTR 的连接。一些芳基磺酰氟在血浆中与 TTR 有效形成缀合物。合成的 11 种 TTR 共价动力学稳定剂在缀合时表现出荧光,因此由于发色团的环境敏感荧光而可能具有成像应用。DOI:10.1021/ja311729d
文献信息
-
Sulfanyl substituted phenyl methanones申请人:Jolidon Synese公开号:US20060149062A1公开(公告)日:2006-07-06The present invention relates to compounds of formula I wherein R 1 , R 2 , R 3 , X, X 1 , and n are as defined in the specification and pharmaceutically acceptable acid addition salts thereof. These compounds are good inhibitors of the glycine transporter 1 (GlyT-1) for the treatment CNS disorders, such as schizophrenia, cognitive impairment, and Alzheimer's disease.
-
Sulfinic Acids and Related Compounds 23. Preparation of Sulfinic Acids by the Reaction of Sulfonyl Halides with Thiols作者:Chew Lee、Lamar FieldDOI:10.1055/s-1990-26884日期:——The reaction of arene- and alkanesulfonyl halides with p-thiocresol in the presence of triethylamine at - 76°C gives triethylammonium sulfinates, which after acidification afford sulfinic acids of 95-100% purity in yields of 51-92% for 18 typical representatives. The synthesis succeeds in certain instances where conventional reduction with aqueous sodium sulfite fails and in other instances often is superior. The method is rapid, mild, selective, convenient, and general, although a few limitations are reported. Characterizations of the sulfinic acids are effected by titration with aqueous sodium nitrite, by IR and 1H-NMR spectra, by preparation of either a S-benzylthiuronium salt or a p-nitrobenzyl ester, and either by elemental analyses or comparison with reported melting points of the acids and derivatives as appropriate.
-
[EN] 1- (2-AMINO-BENZOL) -PIPERAZINE DERIVATIVES AS GLYCINE UPTAKE INHIBITORS FOR THE TREATMENT OF PSYCHOSES<br/>[FR] DERIVES DE 1-(2-AMINO-BENZOL)-PIPERAZINE EN TANT QU'INHIBITEURS DE L'ABSORPTION DU GLYCOCOLLE POUR TRAITER DES PSYCHOSES申请人:HOFFMANN LA ROCHE公开号:WO2005023260A1公开(公告)日:2005-03-17A compound of formula (I) wherein the substituents are described in claim 1 for the treatment of psychoses, pain, neurodegenerative disfunction in memory and learning, schizophrenia, dementia and other diseases in which cognitive processes are impaired, such as attention deficit disorders or Alzheimer's disease.具有式(I)的化合物,其中取代基如权利要求1中所述,用于治疗精神病、疼痛、记忆和学习中的神经退行性功能障碍、精神分裂症、痴呆症以及认知过程受损的其他疾病,如注意力缺陷障碍或阿尔茨海默病。
-
Protein Kinase Inhibitors and Methods for Identiying Same申请人:Lawrence S. David公开号:US20070254312A1公开(公告)日:2007-11-01Inhibitors of protein kinase C (PKC)α, PKCδ and PKCζ are provided which are selective for those PKC isotypes. Combinatorial libraries for identifying protein kinases are also provided, as are methods of identifying protein kinases using those libraries. Additionally, methods of treating a mammal having a deleterious condition, where the condition is dependent on a protein kinase for induction or severity, are provided. Methods of inhibiting protein kinases are also provided.
-
Benzoyl-tetrahydropiperidine derivatives申请人:Jolidon Synese公开号:US20060148797A1公开(公告)日:2006-07-06The present invention relates to compounds of formula I wherein R 1 , R 2 , and Ar; are as defined in the specification. The invention also provides pharmaceutically acceptable acid addition salts thereof and methods for the treatment of neurological and neuropsychiatric disorders, such as schizophrenia, cognitive impairment, and Alzheimer's disease.本发明涉及具有如下式I的化合物,其中R1、R2和Ar如规范中定义的。该发明还提供其药用可接受的酸盐及用于治疗神经和神经精神障碍的方法,例如精神分裂症、认知障碍和阿尔茨海默病。
表征谱图
-
氢谱1HNMR
-
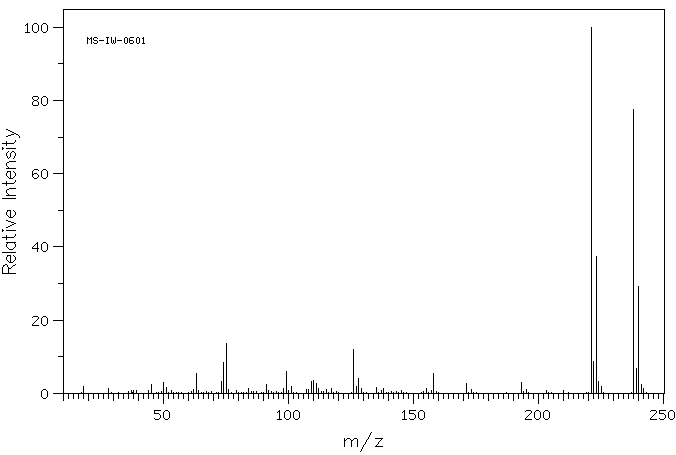
质谱MS
-
碳谱13CNMR
-
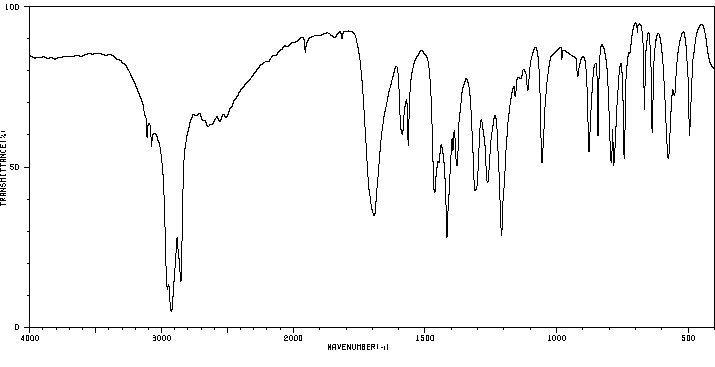
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫