2-methyl-naphtho[1,8-de][1,3]thiazine | 87094-19-7
中文名称
——
中文别名
——
英文名称
2-methyl-naphtho[1,8-de][1,3]thiazine
英文别名
2-Methyl-naphtho[1,8-de][1,3]thiazin;2-Methylnaphtho(1,8-d,E)(1,3)thiazine;3-methyl-2-thia-4-azatricyclo[7.3.1.05,13]trideca-1(12),3,5,7,9(13),10-hexaene
CAS
87094-19-7
化学式
C12H9NS
mdl
——
分子量
199.276
InChiKey
DUDGAFUVQSWQDN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:96.5-97.5 °C
-
沸点:346.4±15.0 °C(Predicted)
-
密度:1.25±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:37.7
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:参考文献:名称:Zakhs; Martynova; Ponyaev, Russian Journal of General Chemistry, 1996, vol. 66, # 8, p. 1349 - 1361摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 sodium acetate 、 四氯化锡 、 溶剂黄146 作用下, 生成 2-methyl-naphtho[1,8-de][1,3]thiazine参考文献:名称:RESEARCHES ON THIAZINES. I. SYNTHESES IN THE PERI-NAPHTHO-META-THIAZINE GROUP摘要:DOI:10.1021/ja01362a020
文献信息
-
Alkylation of the Sulfur- and Nitrogen-containing Compounds Analogous to Thiazoline Systems作者:Yoshio Ohara、Kin-ya Akiba、Naoki InamotoDOI:10.1246/bcsj.56.1508日期:1983.5N,N-Dimethyl-2-(alkylthio)ethylamines and 3-methylthiazolidines underwent N-methylation both with trimethyloxonium tetrafluoroborate (3a) and with methyl iodide. In the methylation of N,N-dimethyl-o-(methylthio)aniline, the main reaction was S-methylation. On the other hand, in the o-ethylthio derivative, S-methylated product was major with methyl iodide, while N-methylated product was major with 3aN,N-二甲基-2-(烷硫基)乙胺和 3-甲基噻唑烷与三甲基氧鎓四氟硼酸盐 (3a) 和甲基碘进行 N-甲基化。N,N-二甲基-邻-(甲硫基)苯胺甲基化反应的主要反应是S-甲基化。另一方面,在邻乙硫基衍生物中,S-甲基化产物以甲基碘为主,而N-甲基化产物以3a为主。在 N-乙基或 N-苄基衍生物中,两种试剂仅发生 S-甲基化。3-甲基-2, 2-二苯基-2,3-二氢-苯并噻唑得到带有 3a 的 S-甲基化产物,表明当 2-取代基体积较大时 S-甲基化变得主要。4-甲基-3,4-dmydro-2H-苯并[b][1,4]噻嗪在氮上用3a甲基化,同时用甲基碘得到S-甲基化产物。吩噻嗪和 1-二烷基氨基-8-(甲硫基)萘仅产生 S-甲基化产物。在 N-甲基化的情况下,选择性 S-甲基化是通过在氮原子上进行质子化,然后是甲基...
-
131. Thiazinocyanines. Part III. Carbocyanines containing the peri-naphtha-1 : 3-thiazine nucleus作者:Frances M. Hamer、Russell J. RathboneDOI:10.1039/jr9430000487日期:——
-
RESEARCHES ON THIAZINES. III. THE SYNTHESIS OF CYANINE DYES OF THE PERI-NAPHTHOMETATHIAZINE SERIES<sup>*†</sup>作者:HOMER van BEUREN JOY、MARSTON TAYLOR BOGERTDOI:10.1021/jo01232a002日期:1936.7
-
OHARA, YOSHIO;AKIBA, KIN-YA;INAMOTO, NAOKI, BULL. CHEM. SOC. JAP., 1983, 56, N 5, 1508-1513作者:OHARA, YOSHIO、AKIBA, KIN-YA、INAMOTO, NAOKIDOI:——日期:——
-
METAL-ASSISTED DELAYED FLUORESCENT MATERIALS AS CO-HOST MATERIALS FOR FLUORESCENT OLEDS申请人:ARIZONA BOARD OF REGENTS ON BEHALF OF ARIZONA STATE UNIVERSITY公开号:US20170267923A1公开(公告)日:2017-09-21A light emitting device includes a first electrode, a hole transporting layer in contact with the first electrode, a second electrode, an electron transporting layer in contact with the second electrode; and an emissive layer between the hole transporting layer and the electron transporting layer. The emissive layer includes a metal-assisted delayed fluorescent (MADF) emitter, a fluorescent emitter, and a host, and the MADF emitter harvests electrogenerated excitons and transfers energy to the fluorescent emitter.
表征谱图
-
氢谱1HNMR
-
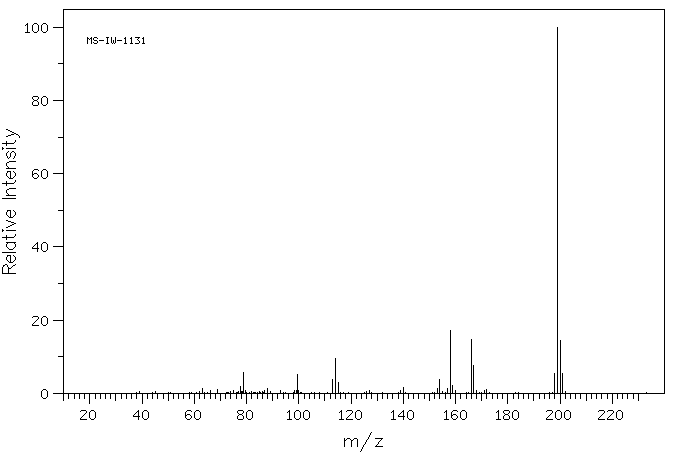
质谱MS
-
碳谱13CNMR
-
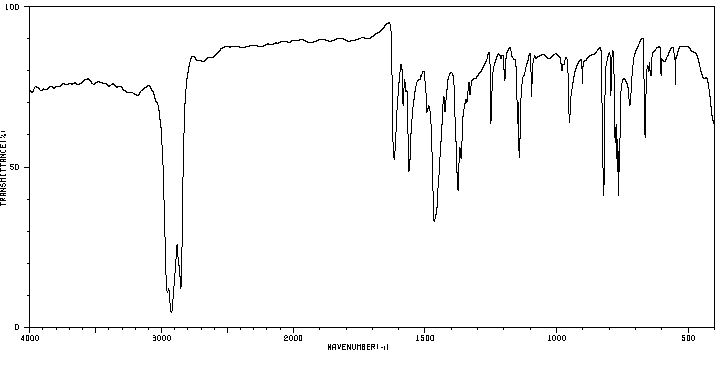
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氟奋乃静
马来酸甲哌丙嗪
马来酸奋乃静
马来酸乙巯拉嗪
锁匹达新
醋酸奋乃静
醋异丙嗪
酒石酸异丁嗪
还原亚甲蓝
达赛马嗪
螺氯丙嗪
莫雷西嗪亚砜
茶氯酸异丙嗪
苹果酸硫乙拉嗪
苯达莫司汀杂质A
苯甲酸2-(2H-1,4-苯并噻嗪-3-基)酰肼
苯甲酸,4-硝基-2-[[3-(三氟甲基)苯基]氨基]-
苯甲酰基氧基甲基-[3-(2-氯吩噻嗪-10-基)丙基]-二甲基氯化铵
苯并噻嗪-5-氧化
苯并噻嗪-5-正离子,3,7-二(二甲氨基)-4-碘-,氯化
苯并噻嗪,10-(2-(4-丙基-1-哌嗪基)丙基)-
苯并[b]吩噻嗪-12-基(苯基)甲酮
苯并[a]吩噻嗪-5-酮
苯丙嗪
苄酰基无色亚甲基兰
芬诺宁
芬乙嗪
舒多昔康
羟乙哌氟嗪
美索哒嗪
美索丙嗪
美洛昔康钾盐
美洛昔康钠
美洛昔康-d3
美洛昔康
美托奋乃酯
美托哌丙嗪酸
美托哌丙嗪
美托咪嗪-d6
美喹他嗪亚砜
美喹他嗪
磺达嗪
硫堇(劳氏紫)
硫利达嗪杂质A(EP)
硫利达嗪N-氧化物
硫利达嗪-5-亚砜
硫利达嗪
硫代哒嗪-d35-亚砜
硫丙拉嗪
盐酸诺美丙嗪