cis-bicyclo<3.2.0>heptane | 278-07-9
中文名称
——
中文别名
——
英文名称
cis-bicyclo<3.2.0>heptane
英文别名
bicyclo[0.2.3]heptane;cis-Bicyclo<3.2.0>heptan;Bicyclo<3,2,0>heptan;Bicyclo[3,2,0]heptan;(1R,5S)-bicyclo[3.2.0]heptane
CAS
278-07-9;5597-72-8
化学式
C7H12
mdl
——
分子量
96.1723
InChiKey
AWYMFBJJKFTCFO-KNVOCYPGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:109.0-109.5 °C
-
密度:0.8522 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:7
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:参考文献:名称:双环[2.2.0]己烷和双环[3.2.0]庚烷的均相重排摘要:自由基从双环[2.2.0]己烷中的桥位和桥头位都提取氢(4)。通过epr光谱法观察到双环[2.2.0]己烷-1-基。双环[2.2.0]己-2-基通过立体电子禁止的β-断裂重排,得到环己-3-烯基。与其他环丁烷不同,化合物(4)与溴原子进行S H 2反应。自由基夺取氢仅从C的亚甲基基团5环双环[3.2.0]庚烷(15A)。通过epr光谱法观察到双环[3.2.0]庚-2-基,以及通过立体电子允许的β-断裂将其重排为2-(环戊-2-烯基)乙基。溴原子从(15a)提取氢,未检测到S H 2反应。使用半经验MINDO / 3和MNDO方法研究了自由基及其重排。DOI:10.1039/p29880001371
-
作为产物:描述:1,6-庚二烯 在 (iPrPDI)Fe(N2)2 作用下, 以 氘代苯 为溶剂, 23.0 ℃ 、50.66 kPa 条件下, 反应 5.0h, 生成 cis-bicyclo<3.2.0>heptane参考文献:名称:Iron-Catalyzed [2π + 2π] Cycloaddition of α,ω-Dienes: The Importance of Redox-Active Supporting Ligands摘要:The bis(imino)pyridine iron bis(dinitrogen) complex, (iPrPDI)Fe(N2)2 (iPrPDI = 2,6-(2,6-iPr2C6H3NCR)2C5H3N), serves as an efficient precursor for the catalytic [2pi + 2pi] cycloaddition of alpha,omega-dienes to yield the corresponding bicycles. For amine substrates, the rate of catalytic turnover increases with the size of the nitrogen substituents, demonstrating competing heterocycle coordination and product inhibition. In one case, a bis(imino)pyridine iron azobicycloheptane product was characterized by X-ray diffraction. Preliminary mechanistic studies highlight the importance of the redox activity of the bis(imino)pyridine ligand to maintain the ferrous oxidation state throughout the catalytic cycle.DOI:10.1021/ja064711u
表征谱图
-
氢谱1HNMR
-
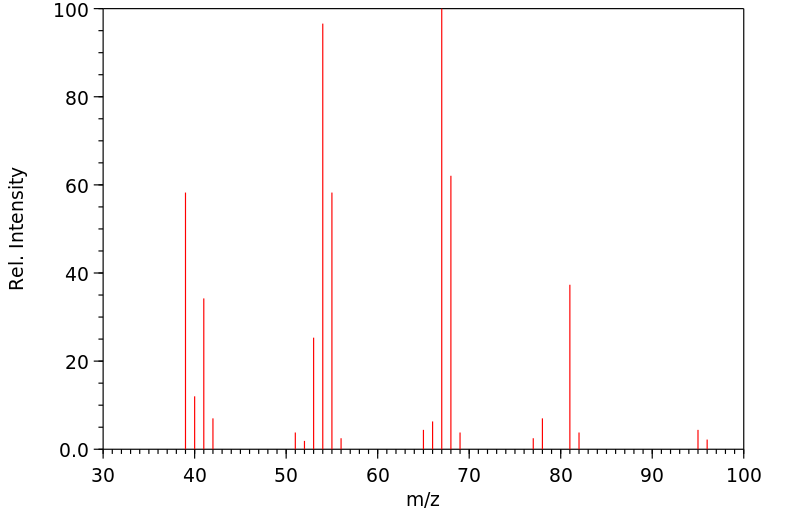
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷