N-(4-nitrobenzoyl)pyrrole | 40123-20-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:323.4±34.0 °C(Predicted)
-
密度:1.30±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.8
-
氢给体数:0
-
氢受体数:3
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4-Amino-phenyl)-pyrrol-1-yl-methanone 853567-11-0 C11H10N2O 186.213
反应信息
-
作为反应物:描述:N-(4-nitrobenzoyl)pyrrole 在 盐酸 、 铁粉 作用下, 以 甲醇 为溶剂, 反应 2.0h, 生成 (4-Amino-phenyl)-pyrrol-1-yl-methanone参考文献:名称:Urea thiadiazole inhibitors of plasminogen activator inhibior-1摘要:揭示了治疗与PAI-1水平升高相关的疾病的方法,包括向需要治疗的患者施用至少一种式(I)的化合物的治疗有效量,或者其药用盐、前药、立体异构体或溶剂化合物,其中:A是芳基或杂芳基,R1-R12在此处定义。该发明还涉及范围在式(I)内的药物组合物和化合物,以及包含式(I)化合物的药物和制造物。公开号:US20050124664A1
-
作为产物:描述:4-nitro-N-(2,4,4-trimethoxybutyl)benzamide 在 喹啉 、 camphor-10-sulfonic acid 作用下, 以 甲苯 为溶剂, 反应 0.5h, 以92%的产率得到N-(4-nitrobenzoyl)pyrrole参考文献:名称:A Practical Preparation of Highly Versatile N-Acylpyrroles from 2,4,4-Trimethoxybutan-1-amine摘要:A novel method for the preparation of N-acylpyrrole is described. The method involves condensation of carboxylic acids with 2,4,4-trimethoxybutan-1-amine, followed by acid-mediated cyclization to form the pyrrole ring. The preparative procedure is highly tolerant of a variety of functional groups.DOI:10.1021/ol3005613
文献信息
-
Synthesis and nuclear magnetic resonance spectroscopic studies of 1-arylpyrroles作者:Chang Kiu Lee、Jung Ho Jun、Ji Sook YuDOI:10.1002/jhet.5570370104日期:2000.1A series of m- and p-substituted 1-phenyl, 1-benzyl, 1-benzoyl, and 1-(2-phenylethyl)pyrroles was prepared and their 1H and 13C nmr spectroscopic characteristics were examined. In general, good correlations were observed between the chemical shift values of the βH and the βC of pyrroles [except 1-(2-phenylethyl)pyrroles] and the Hammettt σ. The observation may be explained in terms of the electronic
-
Ni-catalyzed direct alcoholysis of N-acylpyrrole-type tertiary amides under mild conditions作者:Hang Chen、Dong-Huang Chen、Pei-Qiang HuangDOI:10.1007/s11426-019-9665-5日期:2020.3Abstract N-Acylpyrrole-type amides are a class of versatile building blocks in asymmetric synthesis. We report that by employing Ni(COD)2/2,2′-bipyridine (5 mol%) catalytic system, the direct, catalytic alcoholysis of N-acylpyrrole-type aromatic and aliphatic amides with both primary and secondary alcohols can be achieved efficiently under very mild conditions (rt, 1 h) even at gram scale. By increasing摘要 N-酰基吡咯型酰胺是不对称合成中的一类通用结构单元。我们报告说,通过使用Ni(COD)2 / 2,2'-联吡啶(5 mol%)催化体系,可以有效地实现N-酰基吡咯型芳香族和脂肪族酰胺与伯醇和仲醇的直接催化醇解在非常温和的条件下(室温,1小时),甚至以克为单位。通过将催化剂负载量增加到10 mol%,延长反应时间(18小时)和/或将反应温度提高到50°C / 80°C,该反应可以扩展到络合和受阻的N-酰基吡咯以及N-酰基吡唑类acylindoles,以及其他(功能化的)伯醇和仲醇。在所有情况下,只有1.5当量。使用酒精。该方法的价值已通过其他非对称方法产生的无消旋,手性酰胺催化醇解得到证明。
-
A Convenient Synthesis of<i>N</i>-Acylpyrroles from Primary Aromatic Amides作者:Dallas K. Bates、Anil R. EkkatiDOI:10.1055/s-2003-41020日期:——Synthesis of N-acylpyrroles in 45-85% isolated yield from primary aromatic amides and excess 2,5-dimethoxytetrahydrofuran in presence of one equivalent of thionyl chloride is reported. This method has several advantages including short reaction times, mild reaction conditions, and easy workup. The technique works particularly well for deactivated aromatic amides.据报道,在一当量的亚硫酰氯存在下,由伯芳族酰胺和过量的 2,5-二甲氧基四氢呋喃以 45-85% 的分离产率合成 N-酰基吡咯。该方法具有反应时间短、反应条件温和、易于处理等优点。该技术特别适用于失活的芳族酰胺。
-
General base-catalyzed hydrolysis and carbonyl-<sup>18</sup>O exchange of <i>N</i>-(4-nitrobenzoyl)pyrrole作者:Laurence J Beach、Raymond J Batchelor、Frederick WB Einstein、Andrew J BennetDOI:10.1139/v98-178日期:1998.10.1
Base-promoted hydrolysis kinetics for N-(4-nitrobenzoyl)pyrrole (1) have been measured as a function of buffer concentration at several pH values at 25°C. In addition carbonyl-18O exchange kinetics have been determined at a single pH value (9.48) as a function of 1,4-diazobicyclo[2.2.2]octane (DABCO) concentration. At zero buffer concentration the measured ratio of 18O exchange to hydrolysis (kex/khyd) is approximately 0.04, and this value increases and finally levels off at about 0.23 as the DABCO concentration is increased. These observations are consistent with the buffer acting as a general-base to catalyze both the attack of water to generate an anionic tetrahedral intermediate (To-) and the breakdown of To- to give hydrolysis products.Key words: amide, hydrolysis, catalysis, general-base, tetrahedral intermediate.
-
Dearomative pyrrole (3+2) reaction with geminal bromonitroalkane: synthesis of 2,3-dihydropyrroles作者:Lin Shi、Lidong Liu、Xingyu Lei、Yihan Wang、Yeguang Fang、Peng JiaoDOI:10.1039/d4cc01437e日期:——Dearomative 1,3-dipolar cycloadditions of 1-Boc-pyrroles with in situ generated silver α-bromo alkylidenenitronates delivered a series of 3a,6a-dihydro-4-Boc-pyrrolo[2,3-d]isoxazole-2-oxides (17–91% yields) under very mild conditions. N-Deoxygenation of the cycloaddition product gave a dihydro-pyrrolo[2,3-d]isoxazole, elaborations of which produced various functionalized 2,3-dihydropyrroles and pyrrolidines
表征谱图
-
氢谱1HNMR
-
质谱MS
-
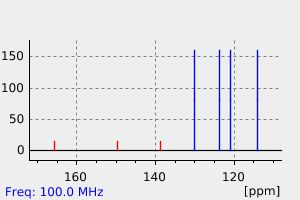
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息