3-(2-萘硫)丙酸 | 1141-45-3
中文名称
3-(2-萘硫)丙酸
中文别名
3-(2-萘基硫代)丙酸
英文名称
3-(2-naphthylthio)propanoic acid
英文别名
3-(naphthalen-2-ylsulfanyl)-propanoic acid;3-(2-naphthylthio)propionic acid;β-<2-Naphthylmercapto>-propionsaeure;β-(2-Naphthylmercapto)-propionsaeure;3--propionsaeure;β-(β-Naphthylmercapto)-propionsaeure;3-(naphthalen-2-ylthio)propanoic acid;3-[2]naphthylsulfanyl-propionic acid;3-[2]Naphthylmercapto-propionsaeure;S-β-Naphthyl-thiohydracrylsaeure;3-naphthalen-2-ylsulfanylpropanoic acid
CAS
1141-45-3
化学式
C13H12O2S
mdl
MFCD00051616
分子量
232.303
InChiKey
XMQJAJQNGNSZFY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104-106°C
-
沸点:232 °C(Press: 12 Torr)
-
密度:1.27±0.1 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,目前没有发现已知的危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:16
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.153
-
拓扑面积:62.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2930909090
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥的地方。同时,确保工作环境具有良好的通风或排气装置。
SDS
上下游信息
反应信息
-
作为反应物:描述:3-(2-萘硫)丙酸 在 对甲苯磺酸 作用下, 以 甲醇 为溶剂, 反应 20.0h, 生成 2,3-dihydro-1H-benzo[f]thiochromen-1-one thiosemicarbazone参考文献:名称:作为组织蛋白酶L抑制剂的硫代苯并二氢吡喃酮硫半碳zone类似物的合成和生化评估。摘要:通过化学合成制备了一系列36个主要在C-6位官能化的包含硫代苯并二氢吡喃酮分子骨架的thiosemicarbazone类似物,并将其评估为组织蛋白酶L和B的抑制剂。该组中最有希望的抑制剂对组织蛋白酶L具有选择性,并显示出IC50值在低纳摩尔范围内。在几乎所有情况下,硫代苯并二氢吡喃酮硫化物类似物均比其相应的硫代苯并二氢吡喃酮砜衍生物具有更好的组织蛋白酶L抑制作用。毫无例外,所评估的化合物对组织蛋白酶B无活性(IC50> 10000 nM)。最有效的组织蛋白酶L抑制剂(IC50 = 46 nM)被证明是6,7-二氟类似物4。小分子已知的结构-活性关系,DOI:10.1021/ml200299g
-
作为产物:参考文献:名称:Fluoride ion-catalyzed conjugate addition for easy synthesis of 3-sulfanylpropionic acid from thiol and α,β-unsaturated carboxylic acid摘要:3-Sulfanylpropionic acids are obtained in excellent yields by proceeding through a simple, mild, and efficient procedure utilizing tetrabutylammonium fluoride (TBAF) as catalyst. (C) 2007 Published by Elsevier Ltd.DOI:10.1016/j.tet.2007.11.064
文献信息
-
Rh-Catalyzed Conjugate Addition of Arylzinc Chlorides to Thiochromones: A Highly Enantioselective Pathway for Accessing Chiral Thioflavanones作者:Ling Meng、Ming Yu Jin、Jun WangDOI:10.1021/acs.orglett.6b02453日期:2016.10.7A highly efficient asymmetric synthesis of chiral thioflavanones is developed via conjugate addition of arylzinc reagents to thiochromones using Rh(COD)Cl2/(R)-3,4,5-MeO-MeOBIPHEP catalyst. This method overcomes catalyst poisoning and substrate inertness and affords a series of chiral thioflavanones (2-arylthiochroman-4-ones) in good yields (up to 91% yield) with excellent ee values (up to 97% ee)
-
Cu-Catalyzed Conjugate Addition of Grignard Reagents to Thiochromones: An Enantioselective Pathway for Accessing 2-Alkylthiochromanones作者:Qingxiong Yang、Jun Wang、Shihui Luo、Ling MengDOI:10.1055/s-0037-1610225日期:2018.9The enantioselective incorporation of alkyl groups in thiochromones was realized for the first time by a Cu/(R,S)-PPF-P t Bu2-catalyzed conjugate addition of Grignard reagents to thiochromones. With this method, a series of 2-methylthiochromanones were obtained in good yields (up to 96% yield) with moderate-to-good ee values (up to 87% ee). The established method expedites the synthesis of a large
-
Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications作者:Shekinah Bass、Dynasty Parker、Tania Bellinger、Aireal Eaton、Angelica Dibble、Kaata Koroma、Sylvia Sekyi、David Pollard、Fenghai GuoDOI:10.3390/molecules23071728日期:——Lithium dialkylcuprates undergo conjugate addition to thiochromones to afford 2-alkylthiochroman-4-ones in good yields. This approach provide an efficient and general synthetic approach to privileged sulfur-containing structural motifs and valuable precursors for many pharmaceuticals, starting from common substrates-thiochromones. Good yields of 2-alkyl-substituted thiochroman-4-ones are attained with
-
Cu(I)-Catalyzed Enantioselective Alkynylation of Thiochromones作者:Ling Meng、Ka Yan Ngai、Xiaoyong Chang、Zhenyang Lin、Jun WangDOI:10.1021/acs.orglett.0c00005日期:2020.2.7A highly efficient asymmetric synthesis of chiral thiochromanones is developed via Cu(I)/phosphoramidite catalyzed asymmetric alkynylation of thiochromones under mild reaction conditions. The catalyst system is tolerant of various thiochromone precursors and terminal alkynes. The established asymmetric transformation provides different enatiomeric-enriched thiochromanones with more molecular complexity
-
Chemical compounds申请人:——公开号:US20030187020A1公开(公告)日:2003-10-02Provided herein are novel and useful compounds having a tryptase inhibition activity, pharmaceutical compositions comprising such compounds, and methods treating subjects suffering from a condition, disease, or disorder that can be ameliorated by the administration of an inhibitor of tryptase, e.g., asthma and inflammatory diseases, to name only a few.本文提供了具有胰蛋白酶抑制活性的新颖和有用化合物,包括含有这些化合物的药物组合物,以及治疗患有可以通过给予胰蛋白酶抑制剂(例如哮喘和炎症性疾病等)而得到改善的疾病、疾病或紊乱的方法。
表征谱图
-
氢谱1HNMR
-
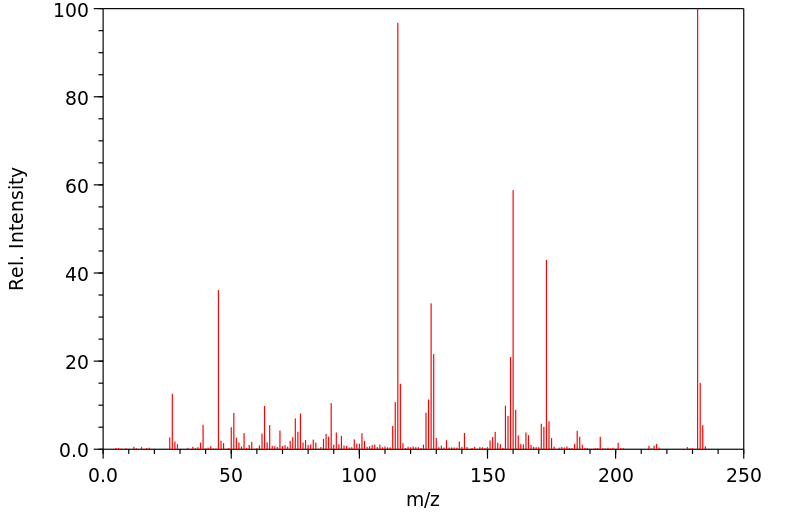
质谱MS
-
碳谱13CNMR
-
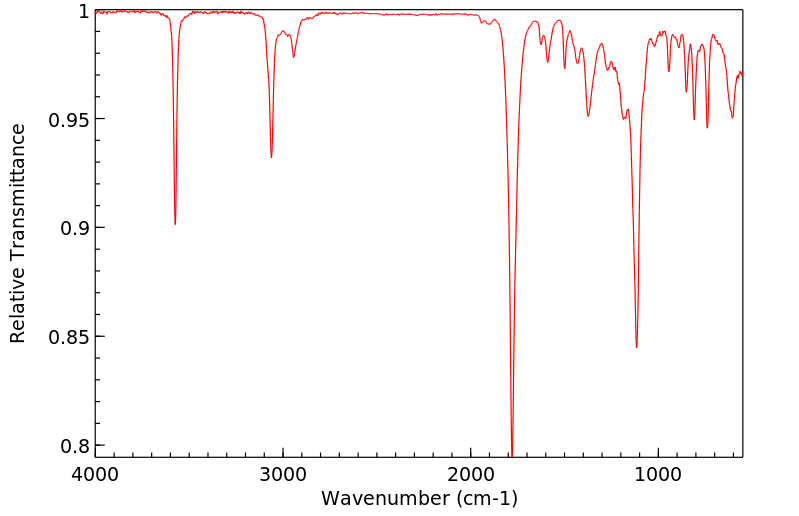
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮