3-[1]萘基-丁酸 | 22339-51-1
中文名称
3-[1]萘基-丁酸
中文别名
——
英文名称
3-(naphthalen-1-yl)butanoic acid
英文别名
β-(1-Naphthyl)-buttersaeure, (+/-)-3-(Naphthyl-1)-buttersaeure;3-<1>Naphthyl-buttersaeure;3-[1]naphthyl-butyric acid;3-[1]Naphthyl-buttersaeure;β-methyl-1-naphthalenepropanoic acid;3-naphthalen-1-ylbutanoic acid
CAS
22339-51-1
化学式
C14H14O2
mdl
——
分子量
214.264
InChiKey
RTYRKKRZQSSAQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108-108.6 °C
-
沸点:378.8±11.0 °C(Predicted)
-
密度:1.159±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.21
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-methyl-3-β-naphthylpropanol 78396-19-7 C14H16O 200.28 4-(萘-1-基)戊-1-醇 4-Naphthalen-1-yl-pentan-1-ol 78396-22-2 C15H18O 214.307
反应信息
-
作为反应物:描述:3-[1]萘基-丁酸 在 吡啶 、 氢氧化钾 、 lithium aluminium tetrahydride 、 氯化亚砜 、 三氟化硼乙醚 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 四氢呋喃 、 水 、 二甲基亚砜 为溶剂, 反应 27.0h, 生成 1-甲基菲参考文献:名称:萘中的亲电取代:萘基丁醇环化成四氢菲摘要:4-(1-萘基)丁醇和4-(2-萘基)丁醇均在回流的三氟化硼-醚中环化,得到1,2,3,4-四氢菲。通过使用相应的1,1-二氘代叔丁醇衍生物并通过220 MHz 1 H nmr光谱分析产物,已证明1-萘丁醇环化有两种不同的途径,(a)通过直接攻击(84%)在在第2位,以及(b)在萘核的1位通过ipso攻击(16%),然后进行重排。2-萘基丁醇仅通过在1-位上的直接取代而环化。另一方面,使用4-(4-甲氧基-1-萘基)-1,1-二氘代丁醇,ipso取代的比例上升到71%,如360 MHz所示1所得四氢菲混合物的H nmr光谱。DOI:10.1039/p29810000286
-
作为产物:参考文献:名称:613.有机反应中的氢化物离子。第二部分。在酸存在下,通过苯乙酮与醌反应制备苯乙铵盐摘要:DOI:10.1039/jr9630003295
文献信息
-
Ligand‐Controlled Regiodivergence in Nickel‐Catalyzed Hydroarylation and Hydroalkenylation of Alkenyl Carboxylic Acids**作者:Zi‐Qi Li、Yue Fu、Ruohan Deng、Van T. Tran、Yang Gao、Peng Liu、Keary M. EngleDOI:10.1002/anie.202010840日期:2020.12.14the ligand environment around the metal center dictates the regiochemical outcome. Markovnikov hydrofunctionalization products are obtained under mild ligand‐free conditions, with up to 99 % yield and >20:1 selectivity. Alternatively, anti‐Markovnikov products can be accessed with a novel 4,4‐disubstituted Pyrox ligand in excellent yield and >20:1 selectivity. Both electronic and steric effects on the
-
Versatile Biocatalytic C(<i>sp</i><sup>3</sup>)−H Oxyfunctionalization for the Site‐ Selective and Stereodivergent Synthesis of α‐ and β‐Hydroxy Acids作者:Yingle Mao、Weijie Zhang、Zunyun Fu、Yanqiong Liu、Lin Chen、Xin Lian、Dan Zhuo、Jiewei Wu、Mingyue Zheng、Cangsong LiaoDOI:10.1002/anie.202305250日期:2023.8.14α-ketoglutarate-dependent aryloxyalkanoate dioxygenases (AADs) are repurposed for applications in biocatalytic oxyfunctionalization. Activity profiling of natural AADs enabled the synthesis of four types of α-and β-hydroxy acids with broad scope, high efficiency, and good selectivity.
-
Biocatalytic process for the production of L-(-)-carnitine from crotonobetaine and strains of Proteeae for use in said process申请人:Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.公开号:EP0457735A1公开(公告)日:1991-11-21A process is described for stereospecifically hydrating crotonobetaine to L-(-)-carnitine via the action of an enzyme produced by four new strains of Proteus mirabilis (NRRL B-18480, NRRL B-18481, NRRL B-18482, and NRRL B-18483) in biotransformation media containing from 10 to 12% (w/v) crotonobetaine inner salt.
-
Synthetic Experiments in the Chrysene Series作者:Louis F. Fieser、Lloyd M. Joshel、Arnold M. SeligmanDOI:10.1021/ja01877a046日期:1939.8
-
Phenanthrene Derivatives. X. Acetylation of 4-Methylphenanthrene作者:W. E. Bachmann、R. O. EdgertonDOI:10.1021/ja01865a086日期:1940.8
表征谱图
-
氢谱1HNMR
-
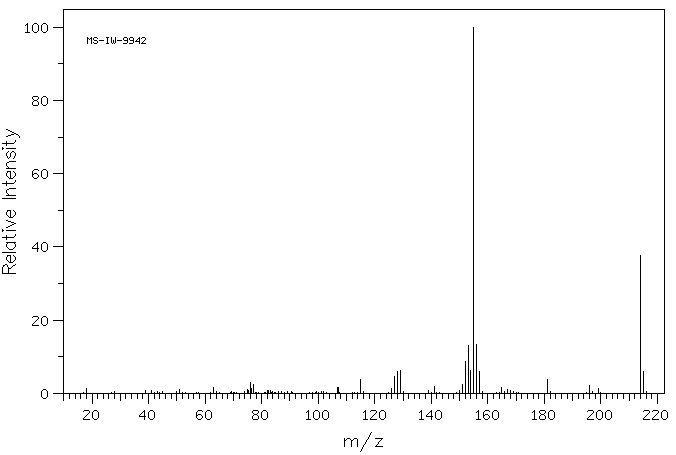
质谱MS
-
碳谱13CNMR
-
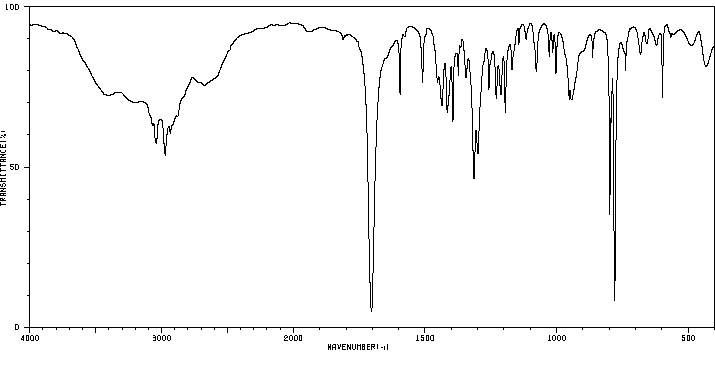
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮