3-硝基-1-萘甲酸 | 4507-84-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:270.5-271.5 °C
-
沸点:445.2±28.0 °C(Predicted)
-
密度:1.468±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:83.1
-
氢给体数:1
-
氢受体数:4
安全信息
-
海关编码:2916399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-硝基-1-萘酸甲酯 methyl 3-nitro-5,6,7,8-tetrahydro-1-naphthoate 13772-63-9 C12H9NO4 231.208 8-溴-3-硝基-1-萘甲酸 3-Nitro-8-brom-1-naphthoesaeure 301337-46-2 C11H6BrNO4 296.077 3-硝基-1,8-萘二甲酸酐 3-nitro-1,8-naphthalic anhydride 3027-38-1 C12H5NO5 243.175 3-硝基萘-1-腈 3-Nitronaphthalene-1-carbonitrile 1016-17-7 C11H6N2O2 198.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-硝基-1-萘酸甲酯 methyl 3-nitro-5,6,7,8-tetrahydro-1-naphthoate 13772-63-9 C12H9NO4 231.208 —— ethyl 3-nitro-1-naphthoate 91901-41-6 C13H11NO4 245.235 —— 3-nitro-1-naphthylcarbinol 79996-96-6 C11H9NO3 203.197 3-氨基-1-萘甲酸 3-aminonaphthalene-1-carboxylic acid 32018-86-3 C11H9NO2 187.198 —— 3-nitro-1-naphthoic acid chloride 54978-07-3 C11H6ClNO3 235.627 —— 3-nitro-1-naphthalenecarboxamide 88575-35-3 C11H8N2O3 216.196 3-氨基-1-萘酸甲酯 methyl 3-amino-1-naphthoate 88790-90-3 C12H11NO2 201.225 —— 3-nitro-1-(bromomethyl)naphthalene 16855-43-9 C11H8BrNO2 266.094 —— N-methoxy-N-methyl-3-nitronaphthalene-1-carboxamide 894789-38-9 C13H12N2O4 260.249
反应信息
-
作为反应物:描述:参考文献:名称:666-15 区域异构体作为 CREB 介导基因转录抑制剂的设计、合成和生物学评价摘要:cAMP 反应元件结合蛋白 (CREB) 是一种核转录因子,与多种人类癌症的发病机制和维持有关。CREB 介导的基因转录的小分子抑制剂的鉴定一直被视为开发癌症疗法的新策略。我们最近发现了一种有效的细胞渗透性 CREB 抑制剂,称为666-15。666-15是一种双萘酰胺,已被证明具有有效的抗乳腺癌活性,且体内无毒性。在本研究中,我们设计并合成了一系列666-15的类似物,以探讨萘环B中区域化学的重要性。这些类似物的生物学评价表明,萘环B中烷氧基和甲酰胺的取代模式对于萘环B中的区域化学至关重要。维持有效的 CREB 抑制活性,表明666-15中独特的生物活性构象至关重要。DOI:10.1016/j.bmcl.2016.12.078
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 盐酸 作用下, 生成 3-硝基-1-萘甲酸参考文献:名称:Leuck; Perkins; Whitmore, Journal of the American Chemical Society, 1929, vol. 51, p. 1835摘要:DOI:
文献信息
-
Dissociation of Naphthoic Acids in Non-Aqueous Media. Comparison of Benzene and Naphthalene Skeletons作者:Patrik Pařík、Jitka Wolfová、Miroslav LudwigDOI:10.1135/cccc20000385日期:——
Seven monosubstituted 1-naphthoic acids were synthesized by new or modified procedures, and their dissociation constants were measured potentiometrically at 25 °C in methanol, acetonitrile, dimethylformamide, and pyridine. Dissociation constants of these along with thirteen substituted 1-naphthoic acids and twenty-five substituted 2-naphthoic acids previously studied were measured at 25 °C in ethanol and dimethyl sulfoxide. The p
K HA values of 3- and 4-substituted 1-naphthoic acids were treated by simple linear regression and principal component analysis, and the results were used for comparison of model compounds and of corresponding 3- and 4-substituted benzoic acids with the aim of comparison of benzene and naphthalene skeletons. It has been found, the 3 and 4 positions of the 1-naphthyl system can roughly be compared with themeta andpara positions of benzene, respectively. -
N-substituted naphthalene carboxamides as neurokinin-receptor antagonists申请人:Astra Zeneca AB公开号:US06365602B1公开(公告)日:2002-04-02A compound of formula I wherein: R is alkyl; R1 is optionally substituted phenyl 2-oxo-tetrahydro-1(2H)-pyrimidinyl, or 2-oxo-1-piperidinyl; R2 is hydrogen, alkoxy, alkanoyloxy, alkoxycarbonyl, alkanoylamino, acyl, alkyl, carbamoyl, N-alkylcarbamoyl, N,N-dialkylcarbamoyl where the alkyl groups are the same or different, hydroxy, thioacyl, thiocarbamoyl, N-alkylthiocarbamoyl, or N,N-dialkylthiocarbamoyl where the alkyl groups are the same or different. X1 and X2 are independently hydrogen or halo, provided that at least one of X1 or X2 is halo; and R3, R4, R5 and R6 are independently hydrogen, cyano, nitro, trifluoromethoxy, trifluoromethyl, or alkylsulfonyl are antagonists of at least one tachykinin receptor and are useful in the treatment of depression, anxiety, asthma, pain, inflammation, urinary incontinence and other disease conditions. Process for their preparation are described, as are compositions containing them and their use.公式I的化合物中:R是烷基;R1是可选择地取代的苯基2-氧代四氢-1(2H)-嘧啶基,或2-氧代-1-哌啶基;R2是氢、烷氧基、烷酰氧基、烷氧羰基、烷酰胺基、酰基、烷基、氨基甲酰基、N-烷基氨基甲酰基、N,N-二烷基氨基甲酰基(其中烷基基团相同或不同)、羟基、硫代酰基、硫代氨基甲酰基、N-烷基硫代氨基甲酰基、或N,N-二烷基硫代氨基甲酰基(其中烷基基团相同或不同)。X1和X2独立地为氢或卤素,但至少X1或X2中的一个为卤素;R3、R4、R5和R6独立地为氢、氰基、硝基、三氟甲氧基、三氟甲基、或烷基磺酰基,它们是至少一种催吐肽受体的拮抗剂,对治疗抑郁症、焦虑症、哮喘、疼痛、炎症、尿失禁和其他疾病情况有用。描述了它们的制备方法,以及含有它们和它们的用途的组合物。
-
Studies directed towards nonyl acridine orange analogues having the potential to act as FRET donors with the PDT drug Pc 4作者:Ping Zhang、Yang Yang、Yun Liu、Myriam E. Rodriguez、Malcolm E. KenneyDOI:10.1039/c5ra28126a日期:——A group of nonyl acridine orange analogues (NAO) was prepared which were designed to have the potential of possessing visible bands allowing them to act in cells as fluorescence resonance energy transfer (FRET) donors with the photodynamic therapy drug Pc 4. The existence of Pc 4-FRET with the analogues of NAO in MCF-7c3 cells was probed. The results suggest that NAO analogues giving strong FRET with制备了一组壬基a啶橙类似物(NAO),其被设计为具有可见带的潜力,使它们可以在光动力疗法药物Pc 4中作为荧光共振能量转移(FRET)供体在细胞中发挥作用。探测了在MCF-7c3细胞中带有NAO类似物的4-FRET。结果表明,可以找到在细胞中具有Pc 4的FAO的NAO类似物。
-
Novel Therapies for Psoriasis作者:Jennifer Cather、Alan MenterDOI:10.2165/00128071-200203030-00003日期:——The T cell-driven immunopathogenesis of psoriasis has been well recognized since cyclosporine first revolutionized the treatment of psoriasis 20 years ago. Almost all investigative and clinical research subsequently, has concentrated on elucidating the specifics of antigen presentation, T cell interaction and the production of specific cytokines. The role of the keratinocyte, previously thought to be the primary target cell in psoriasis pathogenesis, has been relegated to a secondary role and the mechanism of action of systemic methotrexate in psoriasis has been challenged, the primary role of the T lymphocyte is now well known. While psoriasis has traditionally been treated ‘ab initio’ with topical medications (corticosteroids, vitamin D3, and retinoid derivatives), either singly, in combination, or with ultraviolet B (UVB) or psoralens and ultraviolet A (PUVA) therapy, the role of systemic medications has assumed greater prominence. Thus, three systemic medications currently are approved worldwide for the treatment of moderate to severe forms of psoriasis, namely cyclosporine, methotrexate and acitretin. The first two are likely to give significant clearing (greater than 75%) in the majority of cases, whereas acitretin is significantly less effective as monotherapy, but may approach methotrexate and cyclosporine in efficacy, if combined with PUVA or UVB phototherapy. The main limitations of these three drugs remain organ toxicity, especially hepatic toxicity with methotrexate, hypertension and nephrotoxicity with cyclosporine, and teratogenecity and mucocutaneous toxicity with acitretin. Thus, the need for more specific systemic therapy, targeting the T lymphocyte. This has become the major area of clinical research interest over the past 5 years, with the promise of longer-term disease control (improved remissions) and less organ toxicities. Currently, there are over 15 of these ‘biologic’ drugs in various stages of development and clinical trials, either by the subcutaneous, intramuscular or intravenous route. The three main variables are the rapidity of onset, percentages of improvement and remission rates. Without exception, these new systemic agents appear to be remarkably free of systemic organ toxicities (liver, renal, bonemarrow, etc.), with adverse effects being limited to mild flu-like symptoms with the anticipated increase in infections (e.g., herpes simplex) being either equal to placebo or only marginally increased. Not all these agents under evaluation give clinical responses equal to methotrexate or cyclosporine (75% or greater clearing in 75% of cases). In addition, response rates may be slower with some therapies versus others. However, the need for intermittent administration even by the injectable route, longer remissions, lack of systemic organ toxicities and the potential for safer usage in females of child-bearing age, make a compelling argument for widespread acceptance by both patients and the dermatological community. Other modalities under clinical evaluation include vitamin D and retinoid drugs, topically and systemically, with effects on nuclear receptors, as well as more specific wavelengths (308 to 311nm) of UVB phototherapy with application for more localized forms of psoriasis. For the 2 to 3% of the worldwide population of patients with psoriasis the future has never looked brighter.自从20年前环孢素首次彻底改变了牛皮癣的治疗以来,以T细胞驱动的免疫病理机制已被广泛认知。随后的几乎所有研究和临床试验都集中在阐明抗原呈递、T细胞相互作用和特定细胞因子的产生上。角质形成细胞曾被认为是牛皮癣病理机制中的主要靶细胞,但现在其角色已被降为次要,系统性甲氨蝶呤在牛皮癣中的作用机制也受到挑战,T淋巴细胞的主要角色现在已得到了公认。虽然牛皮癣传统上是通过外用药物(如皮质类固醇、维生素D3和类视黄醇衍生物)进行“初始”治疗,单独使用、联合使用,或与紫外线B(UVB)或光敏剂及紫外线A(PUVA)疗法结合使用,但系统性药物的作用变得越来越重要。目前,全球已批准三种系统性药物用于中重度牛皮癣的治疗,即环孢素、甲氨蝶呤和阿维A。前两者在大多数病例中可能给予显著清除(超过75%),而阿维A作为单药治疗的效果明显较差,但如果与PUVA或UVB光疗结合使用,其疗效可能接近甲氨蝶呤和环孢素。这三种药物的主要限制依然是器官毒性,尤其是甲氨蝶呤的肝毒性,环孢素的高血压和肾毒性,以及阿维A的致畸性和黏膜皮肤毒性。因此,针对T淋巴细胞的更特异性系统治疗的需求显得尤为重要。这已经成为过去五年临床研究的主要关注领域,承诺提供更长期的疾病控制(改善的缓解情况)以及更少的器官毒性。目前,已有超过15种“生物”药物在不同开发和临床试验阶段,通过皮下、肌肉或静脉注射给药。三个主要变量是起效的快速性、改善的百分比和缓解率。这些新的系统性药物在器官毒性(肝、肾、骨髓等)方面显然非常少见,副作用仅限于轻度流感样症状,预期的感染增加(如单纯疱疹)则与安慰剂相等或仅稍有增加,并非所有正在评估的药物在临床反应上都能达到甲氨蝶呤或环孢素的水平(在75%的病例中清除达到75%以上)。此外,某些疗法的反应速度可能较慢。然而,即使是通过注射途径的间歇性给药,较长的缓解期,缺乏系统性器官毒性,以及在育龄女性中潜在的更安全使用,使得这些药物得到了患者和皮肤病学界的广泛认可。其他正在临床评估的方法包括维生素D和类视黄醇药物的外用和系统用药,对核受体的影响,以及针对更局部形式的牛皮癣的特定波长(308到311nm)的UVB光疗。对于全球有2%到3%牛皮癣患者的人群来说,未来从未如此光明。
-
Thrombopoietin mimetics申请人:SmithKline Beecham Corporation公开号:US06670387B1公开(公告)日:2003-12-30Non-peptide TPO mimetics are disclosed, as well as a method of treating thrombocytopenia, in a mammal, including a human, in need thereof, which comprises administering to such mammal an effective amount of a selected hydroxy-1-azo-naphthalene derivative.
表征谱图
-
氢谱1HNMR
-
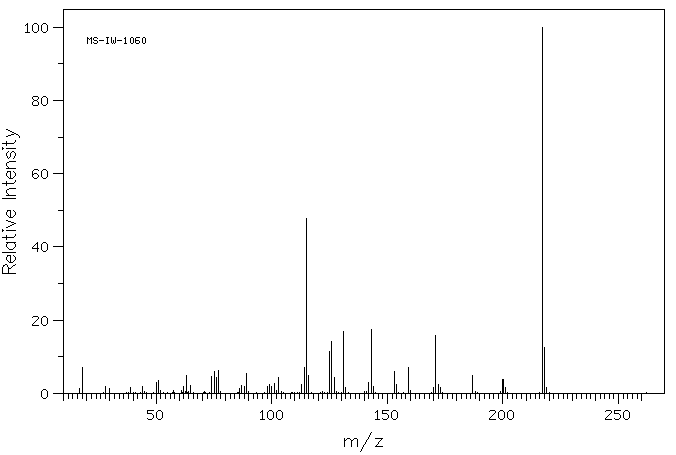
质谱MS
-
碳谱13CNMR
-
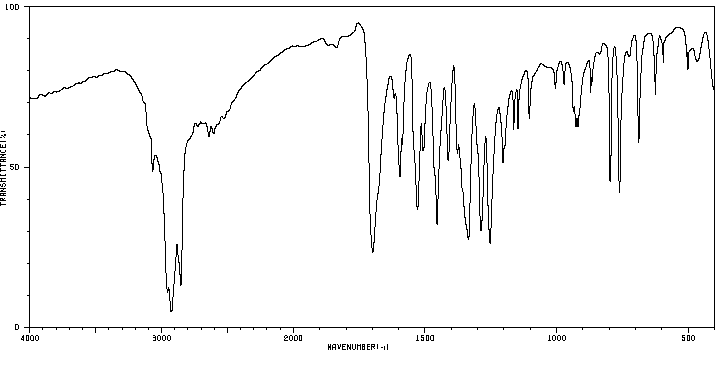
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息