4,4'-[(E)-1,2-乙烯二基]二苯甲腈 | 5216-37-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:272-274 °C
-
沸点:445.0±34.0 °C(Predicted)
-
密度:1.17±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2926909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— cis-4,4'-dicyanostilbene 2510-73-8 C16H10N2 230.269 (E)-4-(对氨基苯)乙烯 (E)-4,4'-diaminostilbene 7314-06-9 C14H14N2 210.279 对甲苯腈 4-methylbenzonitrile 104-85-8 C8H7N 117.15 1-氯-4-[2-(4-氯苯基)乙烯基]苯 (E)-1,2-di(4-chlorophenyl)ethene 5121-74-4 C14H10Cl2 249.139 4,4'-二溴-反-芪 (E)-4,4'-dibromostilbene 18869-30-2 C14H10Br2 338.041 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,4'-二苯甲醛基乙烯 (E)-1,2-bis-(p-phenylaldehyde)ethene 84907-53-9 C16H12O2 236.27 —— Stilbene-4,4'-dicarbonyl Dichloride 52238-17-2 C16H10Cl2O2 305.16
反应信息
-
作为反应物:参考文献:名称:肾上腺皮质激素的合成类似物。摘要:DOI:10.1021/ja01215a014
-
作为产物:描述:参考文献:名称:Synthesis of 4,4'-Dicyanostilbene摘要:DOI:10.1021/ja01258a501
文献信息
-
Oxidative Dephosphorylation of Benzylic Phosphonates with Dioxygen Generating Symmetrical <i>trans</i>-Stilbenes作者:Tianzeng Huang、Tieqiao Chen、Li-Biao HanDOI:10.1021/acs.joc.7b03148日期:2018.3.2Under a dioxygen atmosphere, benzylphosphonates and related phosphoryl compounds can readily produce the corresponding trans-stilbenes in high yields with high selectivity upon treatment with bases. Various functional groups were tolerable under the reaction conditions.
-
Synthesis of novel sulfonyl-stabilized phosphorus ylides, and the kinetics and mechanism of their conventional and flash vacuum pyrolysis reactions作者:Rasha F Al-Bashir、Nouria A Al-Awadi、Osman ME El-DusouquiDOI:10.1139/v05-210日期:2005.9.1
Nine substituted sulfonyl-stabilized phosphorus ylides were prepared by treating their intermediate ylide analogues with phenylmethanesulfonyl fluoride. The stoichiometric ratio of the reactants for each preparation needed to be adjusted according to the basicity of each ylide intermediate. The nine ylide compounds were then subjected to conventional (sealed-tube) gas-phase pyrolysis at 470545 K. The pyrolytic reactions were homogeneous and obeyed a first-order rate equation. The values of the Arrhenius log A (s1) and Ea (kJ mol1) obtained for these reactions averaged 11.12 ± 2.00 and 131.8 ± 24.4, respectively. Analysis of the pyrolysates from conventional pyrolysis and from flash vacuum pyrolysis at 600 K showed the products to be complex mixtures of triphenylphosphine, triphenylphosphine oxide, triphenylphosphine sulfide, and symmetric and unsymmetric alkenes. Conventional pyrolysis also gave novel mixed sulfones and, for the p-methoxyaryl substituent, p-anisaldehyde. The products of the reactions under study are explained on the basis of a mechanism involving a sulfonyl carbene intermediate, and the reaction mechanism is used to rationalize the kinetic results and molecular reactivities.Key words: ylides, synthesis, pyrolysis, kinetics, mechanism.
通过处理它们的中间偶氮化物类似物与苯甲磺酰氟,制备了九种替代磺酰稳定的磷叶类化合物。每种制备所需的反应物的化学计量比需要根据每种叶类中间体的碱性进行调整。然后,这九种叶类化合物经受传统(密封管)气相热解反应,在470-545 K条件下进行。热解反应是均相的,并遵守一级速率方程。对于这些反应获得的阿伦尼乌斯对数A(s-1)和Ea(kJ mol-1)的值分别平均为11.12 ± 2.00和131.8 ± 24.4。从传统热解和600 K的快速真空热解得到的热解产物分析显示,产物是三苯基膦、三苯基膦氧化物、三苯基膦硫化物、对称和非对称烯烃的复杂混合物。传统热解还产生了新型混合磺酮,对于p-甲氧基芳基取代基,产生了对甲醛。根据涉及磺酰卡宾中间体的机制解释了研究中的反应产物,并利用反应机制来解释动力学结果和分子反应性。关键词:叶类化合物,合成,热解,动力学,机制。 -
Effect of Substituents on TiO<sub>2</sub> Photocatalytic Oxidation of <i>trans</i>-Stilbenes作者:Teruyuki Miyake、Yuichiro Hashimoto、Seihou Jinnai、Ryusei Oketani、Suguru HigashidaDOI:10.1246/bcsj.20180223日期:2019.1.15Photocatalytic reaction of trans-stilbene on TiO2 particles produces benzaldehyde with high selectivity in acetonitrile-water mixed solvent. Introduction of electron-donating substituents to the be...
-
Iodine atom-catalysed isomerisation of symmetrically substituted cis-stilbenes作者:W. J. Muizebelt、R. J. F. NivardDOI:10.1039/j29680000913日期:——19 symmetrically substituted cis-stilbenes between 70 and 120°. The reaction rate is of the first order in cis-stilbene derivative and of half order with respect to iodine, which indicates that iodine atoms are the catalytic species. For most compounds value of log k fit a Hammett relation with a negative ρ-value, which can be explained on the basis of the electrophilic character on the iodine atom
-
Diastereoselective 1,3-dipolar cycloadditions of both electronically modified phenyl-nitrile oxides and stilbenes作者:Jan Romanski、Piotr Nowak、Anna Maksymiuk、Christian Chapuis、Janusz JurczakDOI:10.1039/c3ra41718b日期:——stable syn-s-cis conformer in the reaction pathway, given its smaller difference of calculated energies in the corresponding transition states. Such an explanation is further supported by the negative influence of either a polar solvent, stabilizing these more polar transition states (phenyl nitrile oxide in hexane, 76% d.e.; CH2Cl2, 71% d.e.; MeCN, 67% d.e.), or a chelating Lewis acid (MgCl2, 66% d.e.)(E)-1,2-二苯乙烯(12%de)的添加选择性比其对应的(1 R)-8-苯基-薄荷基(38%de)或(2 R)-降冰片烷的薄荷基羧酰腈的选择性低[10,2] sultam(48%de)类似物。当将手性助剂置于亲二氟体上时,也观察到这种较低的选择性,如对-NO 2-苯基腈腈与(1 R)-薄荷醇(4%de)的丙烯酰基衍生物的[3 + 2]环加成反应和(2 R)-硼烷[10,2]杜鹃(60%de)。我们设法通过利用两个假体的Tolbert和Ali合作影响来提高这些非对映选择性,如在(1 R)-薄荷醇(30%de)和( 2 R)-冰片烷[10,2] sultam(98%de)。在该具体情况下Ñ -丙烯酰基莰烷[10,2]磺内酰胺中,我们发现用于电子缺陷的一小预测的负面影响的证据对-取代的苯基腈氧化物上非对映选择性(p -Me 2 N,72%去; p - F,65%de;p- NO 2,60%d
表征谱图
-
氢谱1HNMR
-
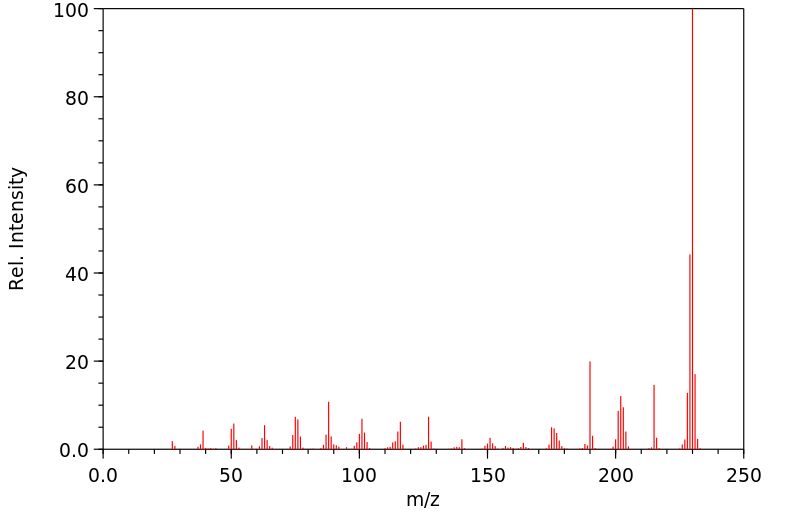
质谱MS
-
碳谱13CNMR
-
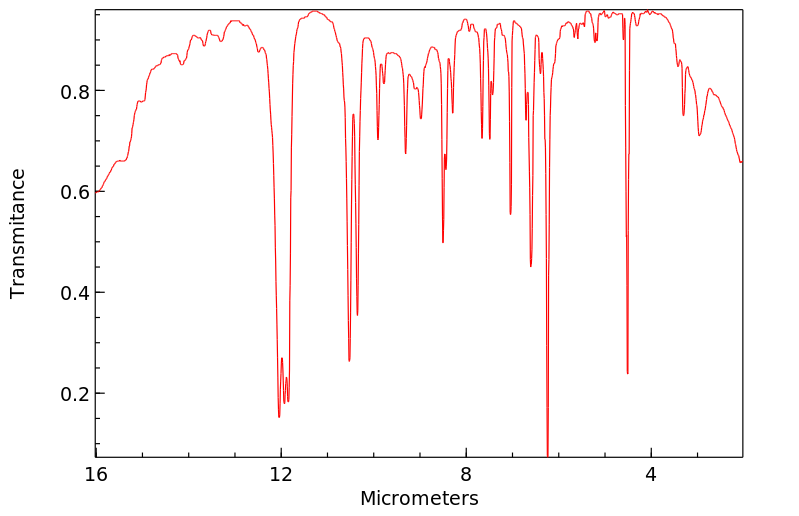
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息