1,4-bis(phenylselenomethyl)benzene | 78808-35-2
中文名称
——
中文别名
——
英文名称
1,4-bis(phenylselenomethyl)benzene
英文别名
p-Bis((phenylseleno)methyl)benzene;1,4-bis(phenylselanylmethyl)benzene
CAS
78808-35-2
化学式
C20H18Se2
mdl
——
分子量
416.283
InChiKey
BCURRNRLLLVKKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.75
-
重原子数:22
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzyl phenyl selenide 18255-05-5 C13H12Se 247.198
反应信息
-
作为反应物:描述:1,4-bis(phenylselenomethyl)benzene 在 sodium tetrahydroborate 作用下, 以 乙醇 为溶剂, 650.0 ℃ 、933.26 Pa 条件下, 生成 benzyl phenyl selenide参考文献:名称:[2.2] 对环戊烷、苯并环丁烯和鳞蝶烯的新合成方法:芳基甲基苯基硒化物的热解摘要:苄基苯基硒化物的快速真空热解以优异的产率得到联苄基和二苯基二硒化物。这种类型的反应成功地应用于标题化合物的合成。DOI:10.1246/cl.1981.627
-
作为产物:参考文献:名称:硒化物的快速热解。联苄、烯烃和相关化合物的合成摘要:研究了一系列硒化物和二硒化物的热解。硒化物和二硒化物与像苄基这样的活性亚甲基结合,以高产率得到各种取代的联苄基和相关的乙烷衍生物。其他二硒化物很容易裂解,以良好的收率与元素硒一起产生各种芳香族和脂肪族烯烃。鳞翅目、[2.2] 对环芳烷和苯并环丁烯是通过热裂解其相应的苯基硒代甲基取代化合物制备的,作为热解的应用。DOI:10.1246/bcsj.55.182
文献信息
-
Microenvironment modulation of cuprous cluster enables inert aryl chlorides activation in single-molecule metallaphotoredox amination作者:Guo-Jin Zha、Wei Ji、Zheng-Hang Qi、Wen-Jie Qiu、Ai-Min Li、Dun-Ru Zhu、Su JingDOI:10.1016/j.jcat.2021.12.007日期:2022.1microenvironment with ferrocenyl and phenyl groups. The cuprous cluster [Cu2I2(L1)] (C1) was proved to be an effective white light-driven SMP, successfully catalyzing the amination of aryl halides with carbazole. This method showed a diverse substrate scopes of aryl halides with electronical and structural variety, affording the highest yield of 63% for 4-chlorobenzonitrile. X-ray crystallographic, in situ electron芳基卤的胺化是有机合成中的重要工具,惰性芳基氯的活化特别困难。我们在此报告了对亚铜簇作为单分子金属光催化剂 ( SMP ) 在 CN偶联反应中直接活化苯氯的芳香微环境调节的首次研究。硒醚L1旨在提供具有二茂铁基和苯基的芳香微环境。亚铜簇 [Cu 2 I 2 ( L1 ) ] ( C1 ) 被证明是一种有效的白光驱动SMP,成功地催化芳基卤化物与咔唑的胺化。该方法显示了具有电子和结构多样性的芳基卤化物的多种底物范围,4-氯苄腈的产率最高,为 63%。X 射线晶体学、原位电子顺磁共振 (EPR) 光谱研究和密度泛函理论 (DFT) 计算表明,C1中的芳香微环境通过SMP和芳基卤化物之间的 π-π 相互作用促进了有效的分子间电子转移。有趣的是,胺化的机制取决于所应用的基材。单电子转移 ( SET ) 和 Cu 2+ 的形成当应用 Ph-I/Ph-Br 时发现了中间体,而当使用 Ph-Cl 底物时,发现了具有
-
HIGUCHI HIROYUKI; SAKATA YOSHITERU; MISUMI SOICHI; OTSUBO TETSUO; OGURA F+, CHEM. LETT., 1981, NO 5, 627-630作者:HIGUCHI HIROYUKI、 SAKATA YOSHITERU、 MISUMI SOICHI、 OTSUBO TETSUO、 OGURA F+DOI:——日期:——
-
HIGUCHI, HIROYUKI;OTSUBO, TETSUO;OGURA, FUMIO;YAMAGUCHI, HACHIRO;SAKATA, +, BULL. CHEM. SOC. JAP., 1982, 55, N 1, 182-187作者:HIGUCHI, HIROYUKI、OTSUBO, TETSUO、OGURA, FUMIO、YAMAGUCHI, HACHIRO、SAKATA, +DOI:——日期:——
表征谱图
-
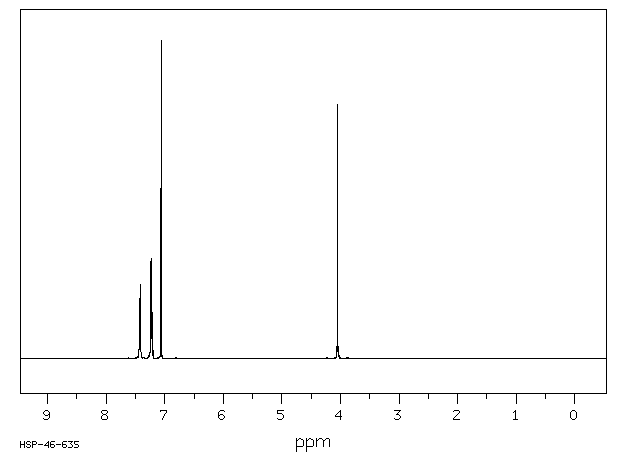
氢谱1HNMR
-
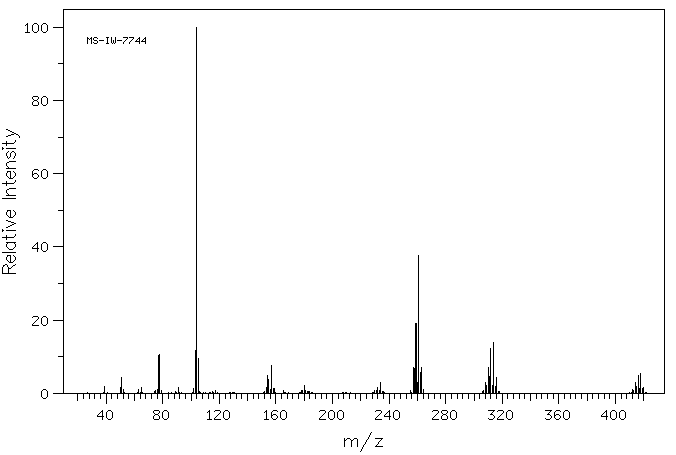
质谱MS
-
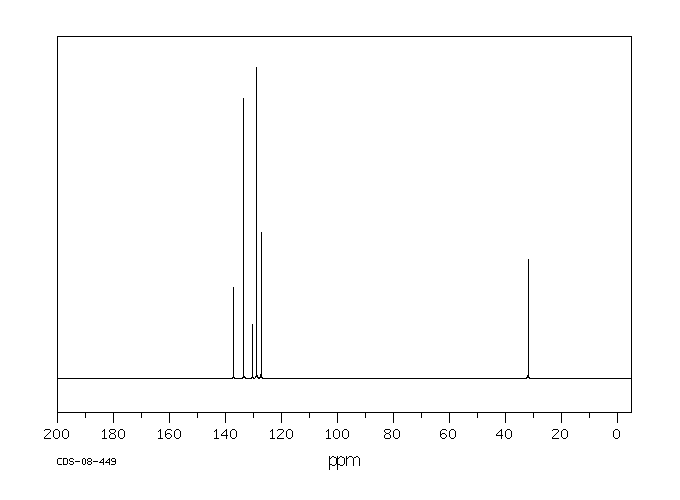
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫