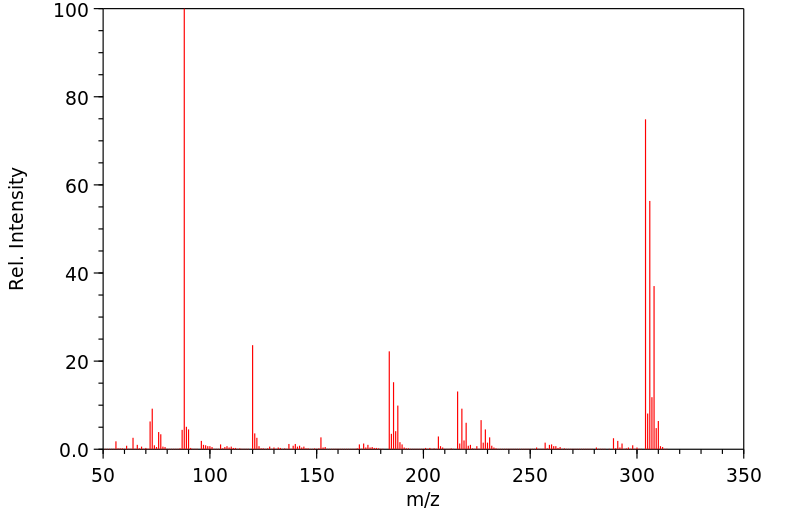
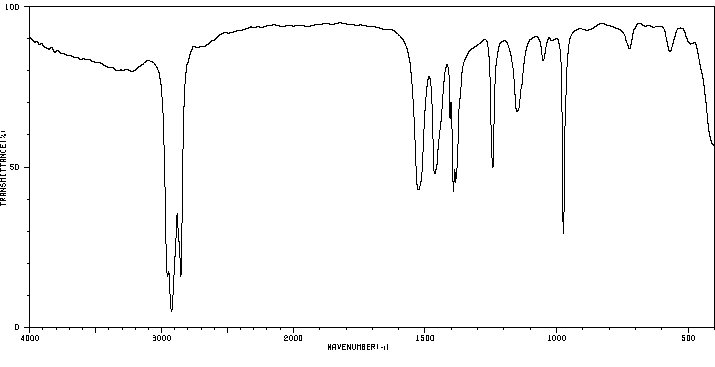
代谢
存在
水溶性代谢物表明在摄入Ziram后胃中产生了二甲二
硫代
氨基甲酸离子。
来源:Hazardous Substances Data Bank (HSDB)
代谢
在口服[35]S ziram后,大鼠通过粪便排除了部分物质(主要是
氯仿可溶形式),但绝大多数以
水溶性代谢物的形式通过尿液排出。24小时后,组织中只留下了少量的浓度,主要是
水溶性形式。除了ziram,通过纸色谱法还区分出了五种
氯仿可溶代谢物和五种
水溶性代谢物。在粪便中,57%的
氯仿可溶活性是未改变的ziram。这五种
氯仿可溶代谢物在胃内容物等位置被找到,这表明但肯定没有证明ziram的部分分解发生在吸收之前。
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠研究中,证实了ziram产生了
二甲基二硫代
氨基甲酸、
二甲胺和
二硫化碳,以及两种不含
锌但含有四个甲基基团的化合物...
来源:Hazardous Substances Data Bank (HSDB)
代谢
水溶性代谢物在大鼠口服放射性标记的
福美锌24小时后,在血液、肾脏、肝脏、卵巢、脾脏和甲状腺中被发现;未改变的
福美锌随粪便排出。
来源:Hazardous Substances Data Bank (HSDB)
代谢
植物中的主要代谢物是
二甲胺基盐的
二甲基二硫代
氨基甲酸;也可以形成四甲基
硫脲、
二硫化碳和
硫。
二甲基二硫代
氨基甲酸可以以DDC-α-
氨基
丁酸和DDC-α-丙
氨酸的形式存在。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
缺
铁性贫血是由于
锌的过量吸收抑制了
铜和
铁的吸收,这很可能是通过肠道粘膜细胞的竞争性结合实现的。
铜和
锌与
铜锌超氧化物歧化酶结合的不平衡
水平与肌萎缩侧索硬化症(ALS)有关。胃酸能溶解
金属
锌,产生腐蚀性的
氯化锌,这可能会损害胃粘膜。
金属烟雾热被认为是对吸入
锌的免疫反应。(L48,L49,A49)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
癌症分类:有致癌性的提示性证据,但不足以评估对人类致癌的可能性;可能对人类有致癌性。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:没有来自人类研究的可用数据。在实验动物中,对齐拉姆(ziram)致癌性的证据有限。总体评估:齐拉姆(Ziram)的致癌性对人不可分类(第3组)。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:对其对人类的致癌性无法分类
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
口服剂量在无油脂的情况下吸收不良。可能通过完好皮肤吸收。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
水溶性代谢物在大鼠口服放射性标记的
福美锌24小时后在血液、肾脏、肝脏、卵巢、脾脏和甲状腺中发现;未改变的
福美锌随粪便排出。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
短期内静态
生物积累实验中...
二甲基二硫代
氨基甲酸锌(ziram).../迅速/通过组织传播。全身积累较低,
生物浓缩因子小于100。全身排泄迅速,45%...的初始放射性...在16天的净化期结束时被保留。色素组织也似乎是主要的分布地点。这可能与这种化合物及其降解产物对
黑色素或与
酪氨酸酶复合的亲和力有关,
酪氨酸酶是一种参与
黑色素合成的含
铜酶。放射性自显影还揭示了甲状腺滤泡的高度标记。结果表明,二
硫代
氨基甲酸盐在各种组织中具有选择性定位,据报道,这些组织是其毒性作用的目标器官。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
ip毒性显著更高的事实表明,口服剂量的吸收相对较慢和/或不完全。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在饮食中添加了2500 ppm的ziram两年的老鼠,肝脏中该化合物的浓度大约为4 ppm。因此,一个每天大约接收30毫克,持续两年的动物,其肝脏中该物质的含量最多只有0.03毫克。相反,储存在骨骼中的
锌含量几乎以与饮食中ziram浓度对数成正比的方式增加,平均值从在饮食中不含和含2500 ppm ziram的情况下维持两年的动物骨骼灰分中的180-300 ppm
锌分别增加。因此,ziram本身并不储存,但由其代谢出的
锌会被轻微储存。
来源:Hazardous Substances Data Bank (HSDB)