5,8-二甲基龙胆山酮酚 | 49599-09-9
中文名称
5,8-二甲基龙胆山酮酚
中文别名
——
英文名称
1,8-dihydroxy-3,5,8-trimethoxyxanthone
英文别名
1-hydroxy-3,5,8-trimethoxy xanthone;1-hydroxy-3,5,8-trimethoxy-xanthone;1-hydroxy-3,5,8-trimethoxyxanthone;1-hydroxy-3,5,8-trimethoxyxanthol;5,8-dimethylbellidifolin;1-hydroxy-3,5,8-trimethoxy-xanthen-9-one;1-Hydroxy-3,5,8-trimethoxyxanthen-9-one
CAS
49599-09-9
化学式
C16H14O6
mdl
——
分子量
302.284
InChiKey
WSRRHFFQNVXIKF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204.5 °C
-
沸点:513.5±50.0 °C(Predicted)
-
密度:1.349±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:22
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:74.2
-
氢给体数:1
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3,5,8-tetramethoxy-9H-xanthen-9-one 54954-13-1 C17H16O6 316.31 1,8-二羟基-3,5-二甲氧基氧杂蒽-9-酮 methylbellidifolin 521-65-3 C15H12O6 288.257 —— 1,5-dihydroxy-3,8-dimethoxyxanthone 134779-25-2 C15H12O6 288.257 雏菊叶龙胆酮 bellidifolin 2798-25-6 C14H10O6 274.23 去甲基雏菊叶龙胆酮 1,3,5,8-tetrahydroxyxanthone 2980-32-7 C13H8O6 260.203 —— 1-glucosyloxy-3-hydroxy-5,8-dimethoxyxanthone 128700-03-8 C21H22O11 450.399 —— 3,5,8-trimethoxyxanthone-1-O-glucopyranoside 119228-03-4 C22H24O11 464.426 —— 1-O-[β-D-xylopyranosyl-(1→6)-β-D-glucopyranosyl]-3,5,8-trimethoxyxanthone 1444411-69-1 C27H32O15 596.542 —— 5,8-dihydroxy-3-methoxy-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-xanthen-9-one 23445-00-3 C20H20O11 436.372 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3,5,8-tetramethoxy-9H-xanthen-9-one 54954-13-1 C17H16O6 316.31
反应信息
-
作为反应物:描述:参考文献:名称:Über einen neuen Bestandteil von Swertia japonica, Makino (Gentianaceae)摘要:DOI:10.1246/bcsj.17.104
-
作为产物:参考文献:名称:Über einen neuen Bestandteil von Swertia japonica, Makino (Gentianaceae)摘要:DOI:10.1246/bcsj.17.104
文献信息
-
Tri- and tetraoxygenated xanthones from Swertia petiolata作者:Khadga S. Khetwal、Binita Joshi、R.S. BishtDOI:10.1016/0031-9422(90)85439-m日期:1990.1Abstract A new tetra-oxygenated xanthone glycoside: 1-glucosyloxy-3-hydroxy-5,8-dimethoxyxanthone has been isolated and identified from the methanolic extract of the aerial parts of Swertia petiolata along with 1,8-dihydroxy-3,5-dimethoxyxanthone; 1,3-dihydroxy-7-methoxyxanthone and 1,7-dihydroxy-3-methoxyxanthone.
-
Studies on the Constituents of Swertia japonica. IV. Isolation and Structure of Xanthones作者:MANKI KOMATSU、TSUYOSHI TOMIMORI、NAOKO MIKURIYADOI:10.1248/cpb.17.155日期:——The chemical constituents of Swertia japonica MAKINO were studied. Three kinds of new natural xanthones, named norswertianin (I), swertianin (III) and methylswertianin (V), were isolated from the whole herb of this plant, together with desmethylbellidifolin (X) and bellidifolin (XII). I was identified as 1, 3, 7, 8-tetrahydroxyxanthone. The direct isolation of I from natural source has never been published, although it has derived from decussatin (VII) and/or swertinin (VIII) by demethylation. The structure of III and V were shown to be 1, 7, 8-trihydroxy-3-methoxyxanthone and 1, 8-dihydroxy-3, 7-dimethoxy-xanthone, respectively. At the same time, "swertianol"was revised the structure to 1, 5, 8-trihydroxy-3-methoxyxanthone, which was proved to be identical with bellidifolin.对獐牙菜MAKINO的化学成分进行了研究。从该植物全草中分离得到三种新的天然呫吨酮,分别命名为norswertianin (I)、swertianin (III)和methylswertianin (V),以及去甲基bellidifolin (X)和bellidifolin (XII)。我被鉴定为1,3,7,8-四羟基氧杂蒽酮。尽管 I 是通过去甲基化从 decussatin (VII) 和/或 swertinin (VIII) 衍生而来,但从未发表过从天然来源直接分离 I 的方法。 III和V的结构分别为1,7,8-三羟基-3-甲氧基呫吨酮和1,8-二羟基-3,7-二甲氧基-呫吨酮。同时,将“獐牙菜醇”的结构修改为1,5,8-三羟基-3-甲氧基呫吨酮,并证明其与桔梗素相同。
-
Studies on the mutagenicity of Swertiae Herba. I. Identification of the mutagenic components.作者:HISAYUKI KANAMORI、IKUNORI SAKAMOTO、MARI MIZUTA、KAZUHISA HASHIMOTO、OSAMU TANAKADOI:10.1248/cpb.32.2290日期:——The mutagenicity of the extract of Swertiae Herba is attributable to its xanthone derivatives. Seven active principles were isolated and six of them were identified as methylbellidifolin, methylswertianin, swertianin, bellidifolin, norswertianin and desmethylbellidifolin, all of which have already been isolated from this plant. The seventh mutagenic compound, a new xanthone derivative formulated as 5, 8-dimethylbellidifolin, was also obtained from methylbellidifolin by methylation with diazomethane as a minor product. The structure-mutagenicity relationship of these compounds, together with some other derivatives, is discussed.
-
Xanthone Glycoside Constituents of <i>Swertia kouitchensis</i> with α-Glucosidase Inhibitory Activity作者:Luo-Sheng Wan、Qiu-Xia Min、Yong-Long Wang、Yao-Dong Yue、Jia-Chun ChenDOI:10.1021/np400082g日期:2013.7.26Ten new xanthone glycosides, kouitchensides A–J (1–10), and 11 known analogues were isolated from an n-butanol fraction of Swertia kouitchensis. The structures of these glycosides were determined on the basis of extensive spectroscopic data interpretation and comparison with data reported in the literature. In an in vitro test, compounds 2, 4, 5, 6, 11, 12, and 13 (IC50 values in the range 126 to 451
-
A xanthone from Swertia chirayita作者:Rakesh K. Asthana、Narendra K. Sharma、Dinesh K. Kulshreshtha、Sunil K. ChatterjeeDOI:10.1016/0031-9422(91)85308-m日期:1991.1Abstract The structure of new xanthone from Swertia chirayita has been established as 1,5-dihydroxy-3,8-dimethoxy xanthone (chiratol) on the basis of spectral and chemical evidence. Two other xanthones, i.e. swerchirin (1,8-dihydroxy-3,5-dimethoxy xanthone) and 7- O -methyl swertianin (1,8-dihydroxy-3,7-dimethoxy xanthone) have been isolated. Swerchirin was identified as the hypoglycaemic principle
表征谱图
-
氢谱1HNMR
-
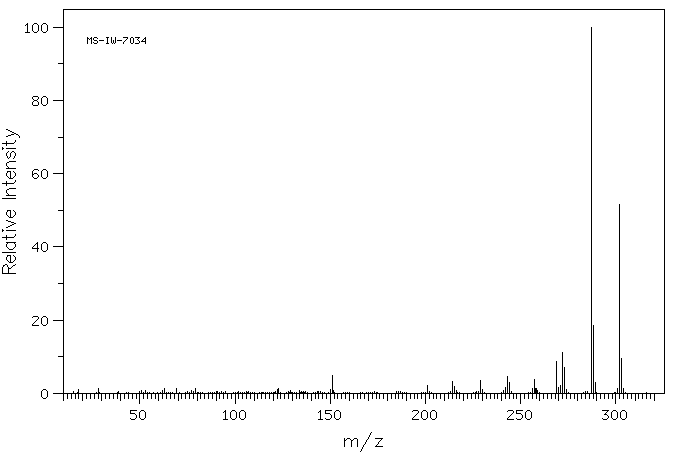
质谱MS
-
碳谱13CNMR
-
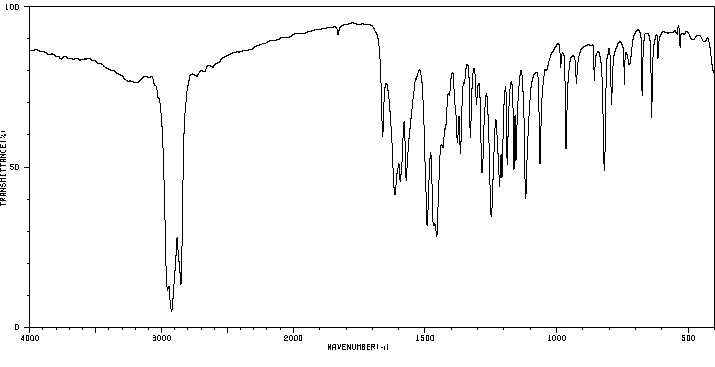
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂