cis-10-(1-propenyl)phenothiazine | 105648-38-2
中文名称
——
中文别名
——
英文名称
cis-10-(1-propenyl)phenothiazine
英文别名
cis-10-Propenylphenothiazine;10H-phenothiazine, 10-[(1Z)-1-propenyl]-;10-[(Z)-prop-1-enyl]phenothiazine
CAS
105648-38-2
化学式
C15H13NS
mdl
——
分子量
239.341
InChiKey
ULYIMBIICQMJOM-FUQNDXKWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34-35 °C(Solv: ethanol (64-17-5))
-
沸点:354.7±25.0 °C(Predicted)
-
密度:1.243±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:28.5
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Anfinogenov, V. A.; Napilkova, O. A.; Sirotkina, E. E., Journal of Organic Chemistry USSR (English Translation), 1987, vol. 23, # 9, p. 1770 - 1772摘要:DOI:
-
作为产物:描述:N-烯丙基吩噻嗪 在 potassium n-butoxide 作用下, 以 二甲基亚砜 、 正丁醇 为溶剂, 反应 0.25h, 以89%的产率得到cis-10-(1-propenyl)phenothiazine参考文献:名称:10-Alkenylphenothiazines. 1. Synthesis and cis,trans-isomerization of 10-propenylphenothiazines摘要:DOI:10.1007/bf00515434
文献信息
-
Monomeric and Polymeric Phenothiazine Photosensitizers for Photoinitiated Cationic Polymerization作者:Zaza Gomurashvili、James V. CrivelloDOI:10.1021/ma0119272日期:2002.4.1of epoxide and vinyl ether monomers is reported. The inclusion of a small amount of the monomeric photosensitizer is effective in markedly accelerating the cationic photopolymerization of the bulk monomer. Polymers prepared by the polymerization of the monomeric phenothiazine photosensitizers also exhibit excellent photosensitization activity when used in combination with diaryliodonium, triarylsulfonium
-
Heterocyclization of N-propenyl-substituted phenothiazines and phenoxazines using electrophiles in an anhydrous medium作者:E. E. Sirotkina、A. I. Khlebnikov、O. A. NapilkovaDOI:10.1007/s10593-009-0219-5日期:2008.1210-Propenylphenothiazine reacts with a catalytic amount of BF3 center dot Et2O in dry ethyl acetate via intramolecular heterocyclization of an intermediate dimeric cation to give mainly 1-ethyl-2-methyl-3-(phenothiazin-10-yl)-2,3-dihydro-1H-pyrido[3,2,1-k,l]phenothiazine and a minor product through fission of phenothiazine which is 1-ethyl-2-methyl-1H-pyrido[3,2,1-k,l]phenothiazine. Under similar conditions 10-propenylphenoxazine gave an oligomer (degree of polymerization 4.4) and the minor product 1-ethyl-2-methyl-1H-pyrido[3,2,1-k,l]phenoxazine likely formed similarly to the phenothiazine analog from the corresponding product of intramolecular heterocyclization (the latter not being observed in the reaction mixture).
-
Synthesis and anticonvulsant activity of cis- and trans-isomers of 10-propenyl- and 10-(phenylvinyl) phenothiazines作者:V. A. Anfinogenov、V. K. Gorshkova、O. A. Napilkova、A. S. Saratikov、V. D. FilimonovDOI:10.1007/bf01145562日期:1987.9
-
XLEBNIKOV, A. I.;ANFINOGENOV, V. A.;FILIMONOV, V. D.;SOKOLOVA, I. V., ZH. ORGAN. XIMII, 25,(1989) N, S. 1547-1553作者:XLEBNIKOV, A. I.、ANFINOGENOV, V. A.、FILIMONOV, V. D.、SOKOLOVA, I. V.DOI:——日期:——
-
XLEBNIKOV, A. I.;ANFINOGENOV, V. A.;SMIRNOVA, E. R., DEROT: ONIITEHXIM G. CHERKASSY 60-XP, 19850113, 101-103作者:XLEBNIKOV, A. I.、ANFINOGENOV, V. A.、SMIRNOVA, E. R.DOI:——日期:——
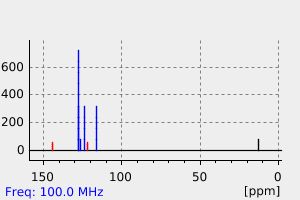
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氟奋乃静
马来酸甲哌丙嗪
马来酸奋乃静
马来酸乙巯拉嗪
锁匹达新
醋酸奋乃静
醋异丙嗪
酒石酸异丁嗪
还原亚甲蓝
达赛马嗪
螺氯丙嗪
莫雷西嗪亚砜
茶氯酸异丙嗪
苹果酸硫乙拉嗪
苯达莫司汀杂质A
苯甲酸2-(2H-1,4-苯并噻嗪-3-基)酰肼
苯甲酸,4-硝基-2-[[3-(三氟甲基)苯基]氨基]-
苯甲酰基氧基甲基-[3-(2-氯吩噻嗪-10-基)丙基]-二甲基氯化铵
苯并噻嗪-5-氧化
苯并噻嗪-5-正离子,3,7-二(二甲氨基)-4-碘-,氯化
苯并噻嗪,10-(2-(4-丙基-1-哌嗪基)丙基)-
苯并[b]吩噻嗪-12-基(苯基)甲酮
苯并[a]吩噻嗪-5-酮
苯丙嗪
苄酰基无色亚甲基兰
芬诺宁
芬乙嗪
舒多昔康
羟乙哌氟嗪
美索哒嗪
美索丙嗪
美洛昔康钾盐
美洛昔康钠
美洛昔康-d3
美洛昔康
美托奋乃酯
美托哌丙嗪酸
美托哌丙嗪
美托咪嗪-d6
美喹他嗪亚砜
美喹他嗪
磺达嗪
硫堇(劳氏紫)
硫利达嗪杂质A(EP)
硫利达嗪N-氧化物
硫利达嗪-5-亚砜
硫利达嗪
硫代哒嗪-d35-亚砜
硫丙拉嗪
盐酸诺美丙嗪