n-pentyl phenyl selenide | 63017-66-3
中文名称
——
中文别名
——
英文名称
n-pentyl phenyl selenide
英文别名
pentyl phenyl selenide;pentyl(phenyl)selane;Pentylselanylbenzene
CAS
63017-66-3
化学式
C11H16Se
mdl
——
分子量
227.208
InChiKey
GHZZLQPVZGFCRV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.62
-
重原子数:12
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Oxone Oxidation of Selenides: A Mild and Efficient Method for the Preparation of Selenones摘要:DOI:10.1021/jo00131a018
-
作为产物:描述:苯硒酚 在 硫酸 、 (rac)-2-(dibenzylamino)-3-phenylpropionaldehyde 作用下, 反应 5.17h, 生成 n-pentyl phenyl selenide参考文献:名称:手性有机锂化合物在添加醛的时间尺度上的构型稳定性摘要:已对α-溴-,α-苯基硒代-和α-苯硫基-烷基-锂化合物1进行了基于动力学拆分的测试,结果表明,将这些物质添加到手性醛6中比对映体的对映体平衡要快。有机锂化合物。DOI:10.1016/s0040-4020(01)90457-0
文献信息
-
<i>p</i>-Selective (sp<sup>2</sup>)-C–H functionalization for an acylation/alkylation reaction using organic photoredox catalysis作者:Ganesh Pandey、Sandip Kumar Tiwari、Bhawana Singh、Kumar Vanka、Shailja JainDOI:10.1039/c7cc07529d日期:——p-Selective (sp2)-C-H functionalization of electron rich arenes have been achieved for acylation and alkylation reaction, respectively, with acyl/alkylselenides by organic photoredox catalysis involving interesting mechanistic pathway.
-
Facile Photochemical Transformation of Alkyl Aryl Selenides to the Corresponding Carbonyl Compounds by Molecular Oxygen: Use of Selenides as Masked Carbonyl Groups作者:Takeshi Hyugano、Suyou Liu、Akihiko OuchiDOI:10.1021/jo801730j日期:2008.11.21groups on the alkyl group were transformed efficiently into the corresponding carbonyl compounds, particularly primary alkyl aryl selenides in good yields, by a simple photolysis in the presence of air or oxygen. This transformation can be conducted without protection of functional groups. The yield of carbonyl compounds was much affected by the solvent viscosity, reaction temperature, concentration
-
一种新型离子型双核席夫碱钛配合物的制备 及其应用申请人:山西医科大学公开号:CN107722049B公开(公告)日:2020-06-30本发明提供了一种一种新型离子型双核席夫碱钛配合物的制备及其应用于催化锌粉还原二硫醚或硒醚制备硫(硒)代酸酯和不对称硫(硒)醚的合成方法。所述的配合物是阳离子型双核席夫碱钛配合物,其中钛原子由氧原子侨连,并与席夫碱配体和一个水分子配位,整个席夫碱钛阳离子部分与阴离子部分形成离子键。以上述双核席夫碱钛配合物作为催化剂,锌粉为还原剂,可还原断裂二硫(硒)醚,进一步分别与酸酐、α‑溴代羰基化合物、溴代烷反应制备硫(硒)代酸酯和不对称硫(硒)醚。本方法克服传统路易斯酸卤化物易潮解,使用无水溶剂,条件苛刻等不足,提供了一种还原二硫醚或硒醚制备硫(硒)代酸酯和不对称硫醚或硒醚的有效路径,具有产率高,操作简便等优点。
-
Air-stable binuclear Titanium(IV) salophen perfluorobutanesulfonate with zinc power catalytic system and its application to C–S and C–Se bond formation作者:Lingxiao Wang、Jie Qiao、Jiancong Wei、Zhiwu Liang、Xinhua Xu、Ningbo LiDOI:10.1016/j.tet.2019.130750日期:2020.1An air-stable μ-oxo-bridged binuclear Lewis acid of titanium(IV) salophen perfluorooctanesulfonate [Ti(salophen)H2O}2O][OSO2C4F9]2 (1) was successfully synthesized by the reaction of TiIV(salophen)Cl2 with AgOSO2C4F9 and characterized by techniques such as IR, NMR and HRMS. This complex was stable open to air over a year, and exhibited good thermal stability and high solubility in polar organic solvents
-
Indium(I) Iodide-Promoted Cleavage of Diaryl Diselenides and Disulfides and Subsequent Condensation with Alkyl or Acyl Halides. One-Pot Efficient Synthesis of Diorganyl Selenides, Sulfides, Selenoesters, and Thioesters作者:Brindaban C. Ranu、Tanmay MandalDOI:10.1021/jo0493727日期:2004.8.1disulfides undergo facile cleavages by indium(I) iodide and the corresponding generated selenate and thiolate anions condense in situ with alkyl or acyl halides present in the reaction mixture. Thus, a simple, efficient, and general procedure has been developed for the synthesis of unsymmetrical diorganyl selenides, sulfides (thioethers), selenoesters, and thioesters by this one-pot reaction at room temperature
表征谱图
-
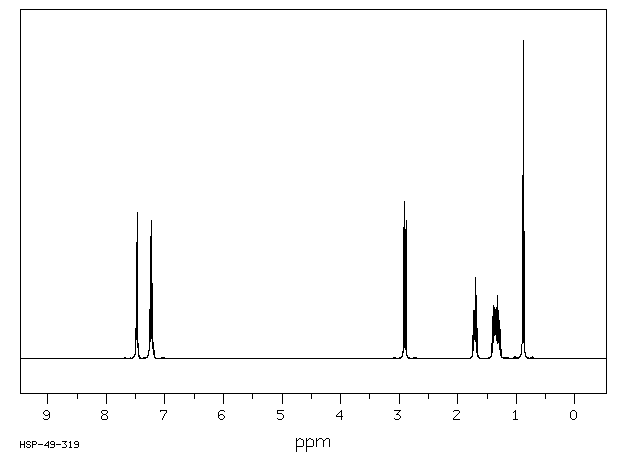
氢谱1HNMR
-
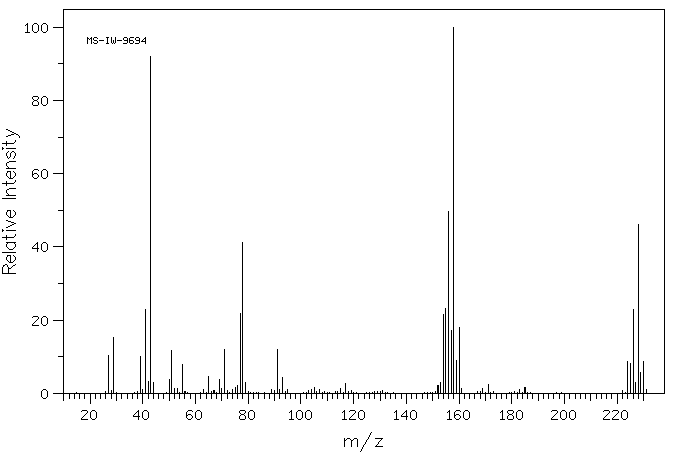
质谱MS
-
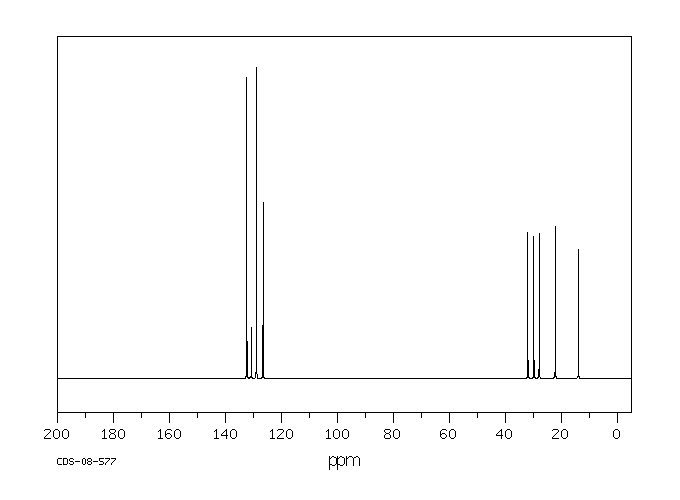
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫