3β-(toluene-4-sulfonyloxy)-5α-cholestane | 3381-52-0
分子结构分类
中文名称
——
中文别名
——
英文名称
3β-(toluene-4-sulfonyloxy)-5α-cholestane
英文别名
3β-hydroxy-5α-cholestane tosylate;3Beta-tosyloxy-5alpha-cholestane;[(3S,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl] 4-methylbenzenesulfonate
CAS
3381-52-0
化学式
C34H54O3S
mdl
——
分子量
542.867
InChiKey
IAEKFZXJTDNGTI-JYFMWEQCSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:136 °C
-
沸点:610.0±24.0 °C(Predicted)
-
密度:1.08±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):11.4
-
重原子数:38
-
可旋转键数:8
-
环数:5.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3β-{[(4-methylphenyl)sulfonyl]oxy}-5α-pregnan-20-one 74578-33-9 C28H40O4S 472.689
反应信息
-
作为反应物:参考文献:名称:雷尼镍对单脂质的脱硫产品。摘要:DOI:10.1021/jo01014a047
-
作为产物:描述:4-甲苯磺酰氰 在 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 间氯过氧苯甲酸 作用下, 以 二氯甲烷 、 氯仿 为溶剂, 生成 3β-(toluene-4-sulfonyloxy)-5α-cholestane参考文献:名称:O-Sulfinylation of alcohols with methanesulfonyl cyanide or p-toluenesulfonyl cyanide.摘要:Reaction of p-toluenesulfonyl cyanide or methanesulfonyl cyanide with alcohols in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or 1,4-diazabicyclo[2.2.2]octane (DABCO) gives sulfinates in good yield. A mechanistic scheme involving sulfinyl cyanates 9 and 21 is suggested.DOI:10.1016/s0040-4020(01)96204-0
文献信息
-
Site-Selective Thiolation of (Multi)halogenated Heteroarenes作者:Frederik Sandfort、Tobias Knecht、Tobias Pinkert、Constantin G. Daniliuc、Frank GloriusDOI:10.1021/jacs.0c01630日期:2020.4.15complementing established methodologies such as nucleophilic aromatic substitution or palladium-catalyzed coupling reactions. Experimental and computational studies suggest a radical chain mechanism with the key step being a homolytic aromatic substitution of the heteroaryl halide by an electrophilic thiyl radical, highlighting an underdeveloped reactivity mode of these radicals.
-
Tris(3,6-dioxaheptyl)amine: A superior complexing agent for dissolving metal reactions作者:Ajay K. Bose、Pietro MangiaracinaDOI:10.1016/s0040-4039(00)95452-2日期:1987.1Commercially available tris(3,6-dioxaheptyl)amine (TDA-1) is an effective phase transfer catalyst for reactions with sodium-potassium alloy in tetrahydrofuran. Deoxygenation of acetates, dehalogenation, and hydrolysis of tosylates and mesylates proceed in high yield. The use of a deuterated alcohol during deoxygenation reactions leads to site-specific deuteration.
-
Modification in the side chain of solomonsterol A: discovery of cholestan disulfate as a potent pregnane-X-receptor agonist作者:Valentina Sepe、Raffaella Ummarino、Maria Valeria D'Auria、Gianluigi Lauro、Giuseppe Bifulco、Claudio D'Amore、Barbara Renga、Stefano Fiorucci、Angela ZampellaDOI:10.1039/c2ob25800e日期:——Seven synthetic analogues of the PXR (pregnane-X-receptor) potent natural agonist solomonsterol A were prepared by total synthesis. Their activity toward PXR was assessed by transactivation and RT-PCR assays. The study discloses cholestan disulfate (8) as a new, simplified agonist of PXR. By in vitro studies on hepatic cells we have demonstrated that this compound is a potent PXR agonist and functional characterization in human macrophages and hepatic stellate cells provided evidence that cholestan disulfate (8) has the ability to modulate the immune response triggered by bacterial endotoxin as well as to counter-activate hepatic stellate cell activation induced by thrombin. Because inhibition of immune-driven circuits might have relevance in the treatment of inflammation and liver fibrosis, the present data support the development of cholestan disulfate (8) in preclinical models of inflammatory diseases.
-
Steroidal sulphur compounds. Part II. Pyrolysis, absolute configuration, and optical rotatory dispersion of steroidal methyl sulphoxides作者:D. Neville Jones、M. J. Green、M. A. Saeed、R. D. WhitehouseDOI:10.1039/j39680001362日期:——sulphur in 3β-acetoxy-(R)- and (S)-5α-methylsulphinylcholestane was also determined by an interpretation of intramolecular reactions involving the sulphoxide group. Two Cotton effects of opposite sign near 210 and 230 mµ occur in the o.r.d. spectra of the sulphoxides. Their relevance to chirality at sulphur and to restricted rotation about a C–S bond is discussed.
-
Stereospecific Nickel-Catalyzed Reductive Cross-Coupling of Alkyl Tosylate and Allyl Alcohol Electrophiles作者:Quentin D. Tercenio、Erik J. AlexanianDOI:10.1021/acs.orglett.1c02616日期:2021.9.17The stereospecific cross-coupling of easily accessed electrophiles holds significant promise in the construction of C–C bonds. Herein, we report a nickel-catalyzed reductive coupling of allyl alcohols with chiral, nonracemic alkyl tosylates. This cross-coupling delivers valuable allylation products with high levels of stereospecificity across a range of substrates. The catalytic system consists of
表征谱图
-
氢谱1HNMR
-
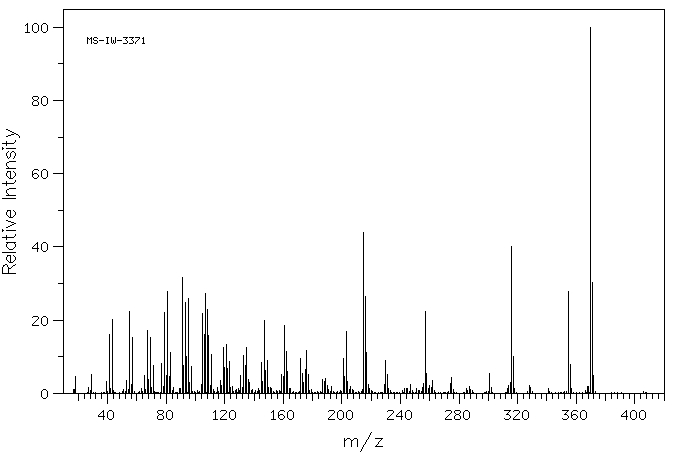
质谱MS
-
碳谱13CNMR
-
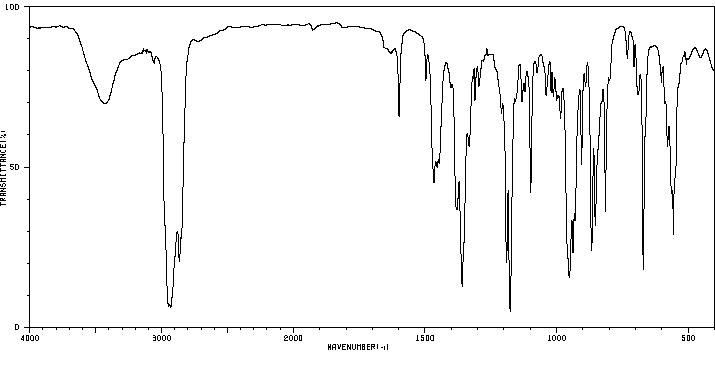
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B